Haemoglobinopathy Diagnosis
Barbara J. Bain
- English
- ePUB (disponibile sull'app)
- Disponibile su iOS e Android
Haemoglobinopathy Diagnosis
Barbara J. Bain
Informazioni sul libro
An updated, essential guide for the laboratory diagnosis of haemoglobin disorders
This revised and updated third edition of Haemoglobinopathy Diagnosis offers a comprehensive review of the practical information needed for an understanding of the laboratory diagnosis of haemoglobin disorders. Written in a concise and approachable format, the book includes an overview of clinical and laboratory features of these disorders. The author focuses on the selection, performance, and interpretation of the tests that are offered by the majority of diagnostic laboratories. The book also explains when more specialist tests are required and explores what specialist referral centres will accomplish. The information on diagnosis is set in a clinical context.
The third edition is written by a leading haematologist with a reputation for educational excellence. Designed as a practical resource, the book is filled with illustrative examples and helpful questions that can aide in the retention of the material presented. Additionally, the author includes information on the most recent advances in the field. This important text:
• Contains a practical, highly illustrated, approach to the laboratory diagnosis of haemoglobin disorders
• Includes "test-yourself" questions and provides an indispensable tool for learning and teaching
• Presents new material on antenatal screening/prenatal diagnostic services
• Offers myriad self-assessment case studies that are ideal for the trainee
Written for trainees and residents in haematology, practicing haematologists, and laboratory scientists, Haemoglobinopathy Diagnosis is an essential reference and learning tool that provides a clear basis for understanding the diagnosis of haemoglobin disorders.
Domande frequenti
Informazioni
1
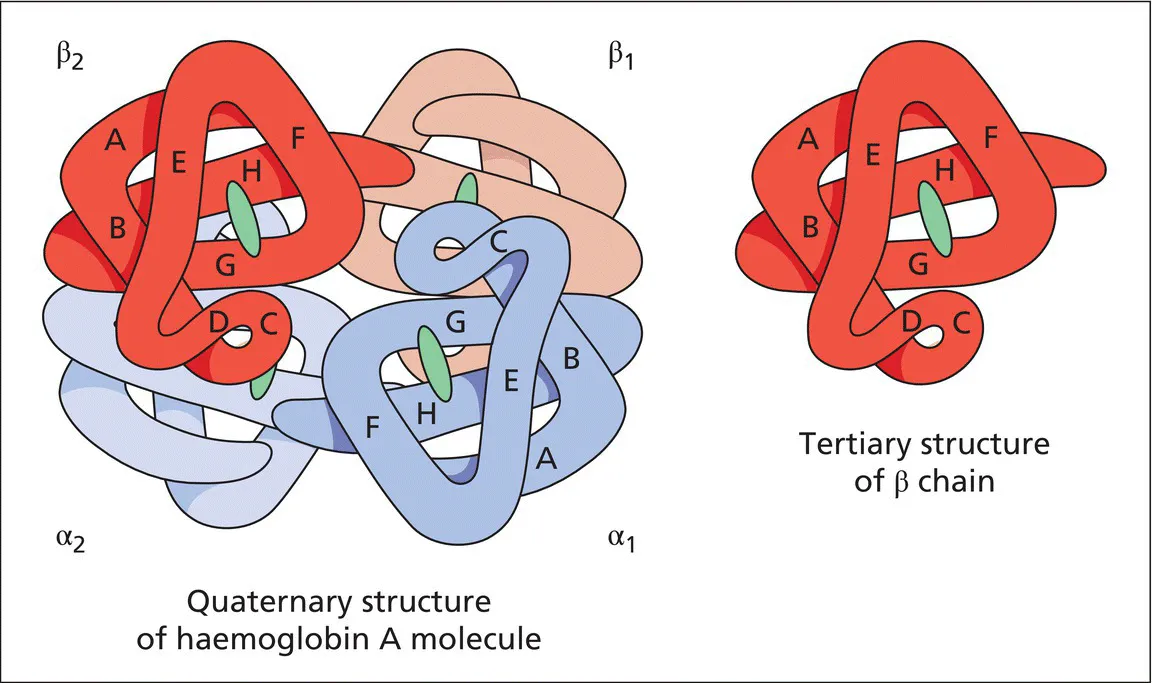
Haemoglobin and the genetics of haemoglobin synthesis
Haemoglobins and their structure and function