
eBook - ePub
Metabolic Effects Of Dietary Fructose
Sheldon Reiser
This is a test
Condividi libro
- 176 pagine
- English
- ePUB (disponibile sull'app)
- Disponibile su iOS e Android
eBook - ePub
Metabolic Effects Of Dietary Fructose
Sheldon Reiser
Dettagli del libro
Anteprima del libro
Indice dei contenuti
Citazioni
Informazioni sul libro
It is hoped that the material presented in this book will provide the reader with a detailed description of the published research pertaining to the metabolic effects of dietary fructose, will define future research needs, and will stimulate interest in further research aimed at evaluating the advisability of the intake of fructose by humans.
Domande frequenti
Come faccio ad annullare l'abbonamento?
È semplicissimo: basta accedere alla sezione Account nelle Impostazioni e cliccare su "Annulla abbonamento". Dopo la cancellazione, l'abbonamento rimarrà attivo per il periodo rimanente già pagato. Per maggiori informazioni, clicca qui
È possibile scaricare libri? Se sì, come?
Al momento è possibile scaricare tramite l'app tutti i nostri libri ePub mobile-friendly. Anche la maggior parte dei nostri PDF è scaricabile e stiamo lavorando per rendere disponibile quanto prima il download di tutti gli altri file. Per maggiori informazioni, clicca qui
Che differenza c'è tra i piani?
Entrambi i piani ti danno accesso illimitato alla libreria e a tutte le funzionalità di Perlego. Le uniche differenze sono il prezzo e il periodo di abbonamento: con il piano annuale risparmierai circa il 30% rispetto a 12 rate con quello mensile.
Cos'è Perlego?
Perlego è un servizio di abbonamento a testi accademici, che ti permette di accedere a un'intera libreria online a un prezzo inferiore rispetto a quello che pagheresti per acquistare un singolo libro al mese. Con oltre 1 milione di testi suddivisi in più di 1.000 categorie, troverai sicuramente ciò che fa per te! Per maggiori informazioni, clicca qui.
Perlego supporta la sintesi vocale?
Cerca l'icona Sintesi vocale nel prossimo libro che leggerai per verificare se è possibile riprodurre l'audio. Questo strumento permette di leggere il testo a voce alta, evidenziandolo man mano che la lettura procede. Puoi aumentare o diminuire la velocità della sintesi vocale, oppure sospendere la riproduzione. Per maggiori informazioni, clicca qui.
Metabolic Effects Of Dietary Fructose è disponibile online in formato PDF/ePub?
Sì, puoi accedere a Metabolic Effects Of Dietary Fructose di Sheldon Reiser in formato PDF e/o ePub, così come ad altri libri molto apprezzati nelle sezioni relative a Medicine e Nutrition, Dietics & Bariatrics. Scopri oltre 1 milione di libri disponibili nel nostro catalogo.
Informazioni
Chapter 1
FRUCTOSE OCCURRENCE IN FOODS AND PRODUCTION OF HIGH-FRUCTOSE CORN SWEETENERS
I. Characteristics
Fructose, also called levulose and fruit sugar, is a naturally occurring hexose. It is found in berries and other fruits, and comprises approximately 50% of honey and sucrose. Dubrunfaut isolated pure fructose in 1847.1 In 1874, fructose was reported to be better tolerated by diabetics than sucrose or glucose.2 It has been used as a sweetener in special foods for diabetics since that time. β-D-Fructofuranose is the only form of fructose which is fermented by yeast. Fructopyranose is the only known crystalline form of fructose and is probably the sweetest naturally occurring sugar. Fructose is more soluble in water than is sucrose. A saturated sugar solution of 100 g at 25°C will contain 81 g of fructose, 67 g of sucrose, and 51 g of glucose.3 It is the difference in this property, solubility, which makes sucrose the more popular sugar for use in certain products such as candies and frostings, and fructose more popular in beverages or as a sweetener in canned fruits. The high solubility of fructose makes it more hydroscopic and therefore candies or frostings which contain a high level of fructose may become more sticky or gooey than is desirable. Relative humidity changes of only 1% can alter the consistency of candy containing a high percentage of fructose.
Fructose has been reported to be 15 to 80% sweeter than sucrose,4 but sweetness is dependent on pH, temperature, and concentration. A fructose solution is sweetest when it is dilute, cool, and slightly acidic, making it a useful sweetener in soft drinks.5 In water, fructose can be detected at lower levels than sucrose; however, fructose in pear nectar was perceived as less sweet than sucrose.6 Fructose cake was judged less sweet than sucrose cake, probably due to the browning reaction.7
II. Occurrence
Fructose is the predominant monosaccharide in a number of fruits (Table 1) including apples, cherries, currants, and pears.8 Fructose, as a component of sucrose, also occurs in considerable amounts in many fruits. According to U.S. Department of Agriculture information, simple carbohydrate from fruits provides 10 to 15% of total sugar intake.9 From 1913 to 1969 the percentage of simple carbohydrate as fructose declined from 4 to 2% because the consumption of fresh apples, which are a good source of fructose, decreased from 60 to 16 lb per capita. During this same period there was an increase in consumption of citrus fruits, but since they contain less fructose than apples, total fructose intake was still lower. Fresh apple consumption in 1980 was approximately the same as in 1969. Fructose was reported to supply 7.4% of total simple carbohydrate intake of 214 g/day. Application of various herbicides was reported to alter simple sugar content of apples, blueberries, and peaches, but the differences in fructose were minimal.10 Values for fructose in the edible portion of apples ranged from 6.5 to 8.3%, of blueberries from 5.4 to 6.1%, and of peaches from 0.6 to 0.7%. Sucrose content in the edible portion of these fruits ranged from 4.1 to 4.9% in apples, from 0.8 to 1.5% in blueberries, and from 6.1 to 6.8% in peaches.
There are a number of vegetables which contain measurable amounts of fructose, both free and as a component of sucrose (Table 2). Most vegetables contain 1 to 2% free fructose and up to 3% fructose as sucrose.11 While this may not seem much, it may constitute a high percentage of the total number of calories of these vegetables. Inulin, which is a polymer of fructose (Figure 1), is present in chicory, sweet potatoes, and Jerusalem artichokes. Inulin may be hydrolyzed to fructose at high temperatures under acid conditions. Fructose is also present in some legumes as the trisaccharide raffinose (Figure 2) and the tetrasaccharide stachyose (Figure 3). Raffinose contains one molecule each of galactose, glucose, and fructose. Stachyose contains two galactose molecules and one each of glucose and fructose. These compounds are not digested in the human small intestine. They are, however, fermented in the large intestine, and may be the source of flatulence which occurs after ingestion of legumes. A distribution of various sugars and these fructose-containing oligosaccharides occurring in legumes appears in Table 3.
Table 1
AMOUNTS OF SUGARS IN FRUITS
AMOUNTS OF SUGARS IN FRUITS
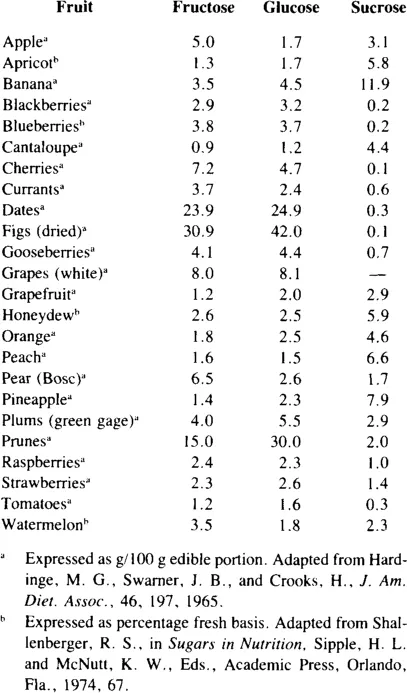
Table 2
AMOUNTS OF SUGARS IN VEGETABLES
AMOUNTS OF SUGARS IN VEGETABLES
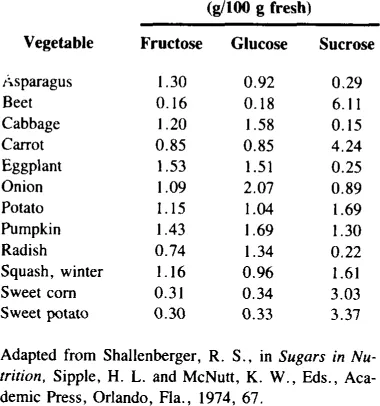
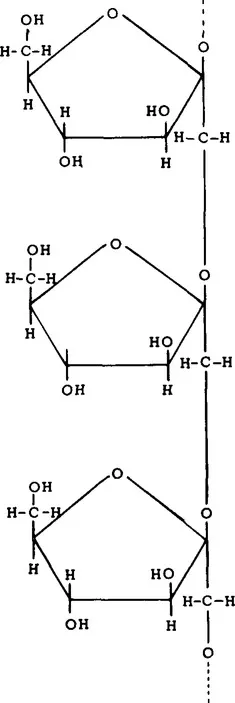
FIGURE 1. Structure of inulin, a polymer of β-D-fructofuranose linked by 2,1 bonds.
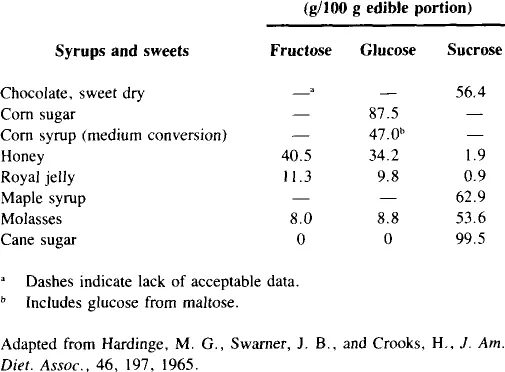
Fructose occurs either free or as a component of sucrose in a variety of sweets (Table 4). Honey contains slightly more fructose than glucose, corn sugar is almost completely glucose, cane and maple sugar are predominantly sucrose, and molasses contains mostly sucrose, but also is about 8% fructose.
Although honey provides the highest concentration of fructose as a natural sweetener, relatively small amounts of it are consumed. Honey consumption per capita from the period 1960 to 1981 averaged about 1 lb/year with a range of 0.7 to 1.2 lb.12 Edible syrup consumption (another minor source of fructose) during this same period declined 50% from only 0.8 lb per capita in 1960 to a steady 0.4 lb from 1974 to 1981. Beet sugar consumption ranged from 24.5 to 32.0 lb per capita.
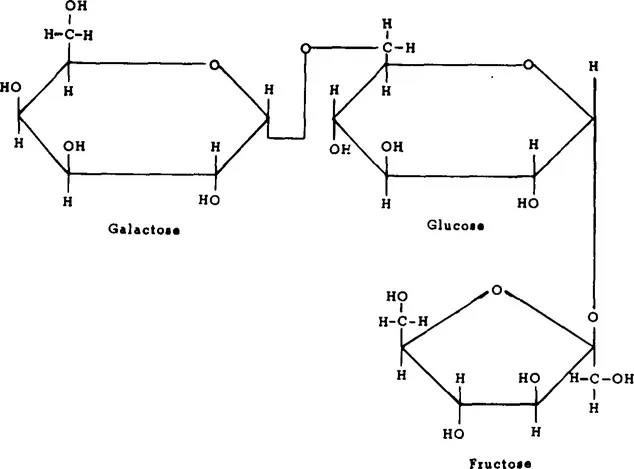
FIGURE 2. Structure of raffinose, α-D-galactopyranosy1-(1,6)-α-D-glucopyranosyl-(1,2)-β-D-fructofuranoside.
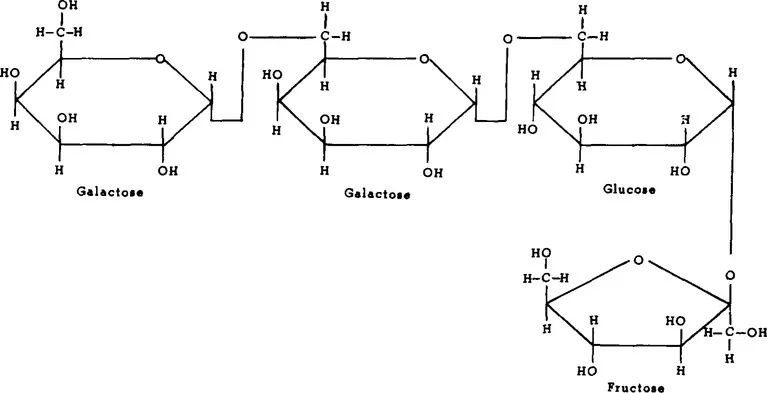
FIGURE 3. Structure of stachyose, α-D-galactopyranosyl-(1,6)-α-D-galactopyranosyl-(1,6)-α-D-glucopyranosyl-(1,2)-β-D-fructofuranoside.
III. High-Fructose Corn Sweeteners
A. Production
The two major sources of fructose in the U.S. diet are cane sugar and high-fructose corn sweeteners. Cane sugar consumption averaged approximately 100 lb per capita per year from 1960 to 1974.9 Cane sugar is produced from stalks of sugar cane from which the leaves have been removed. Heavy rollers crush these stalks to express the juice.13 The 10 to 15% sugar juice is treated with lime and filtered to remove impurities. This filtered solution is then evaporated until concentrated enough for crystallization. The crystals that are formed are covered with molasses. The removal of the molasses and further purification to produce white granulated sugar is called “refining”. Crystals are washed, centrifuged to remove the molasses, and then dissolved in warm water, and most remaining impurities are removed by filtration or precipitation. Charcoal is used to remove the last remaining impurities. New crystals are then formed in another evaporation under a vaccuum at low temperature, and are separated according to size. Beet sugar is a minor source of sucrose made by the same general process, except that in the first step sugar is extracted with hot water rather than by crushing.
Table 3
SUGARS AND OLIGOSACCHARIDES CONTAINING FRUCTOSE IN VARIOUS LEGUMES
SUGARS AND OLIGOSACCHARIDES CONTAINING FRUCTOSE IN VARIOUS LEGUMES
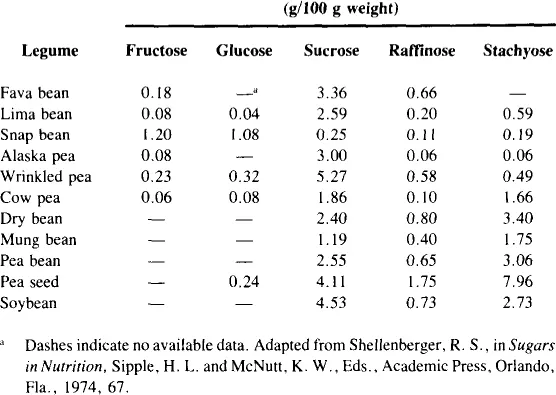
Table 4
AMOUNTS OF SUGARS IN SYRUPS AND SWEETS
AMOUNTS OF SUGARS IN SYRUPS AND SWEETS
The introduction of high-fructose corn sweeteners in 1967 has led to their steady replacement of cane sugar in many processed foods.13 In 1981 cane sugar consumption was less than 80 lb per capita and high-fructose corn sweeteners had increased to about 20 lb.
There are three major high-fructose corn sweeteners used in processed foods in this country. They contain 42, 55, or about 90% fructose. The first mass-produced high-fructose corn sweeteners contained only about 15% fructose, with the remainder being dextrins or more complex glucose polymers. In 1968, 42% fructose was produced in a batch process and in 1973 in a continuous process. In 1977, high-fructose corn sweetener containing 55% fructose was introduced by combining 90% fructose with 42% fructose corn sweetener.
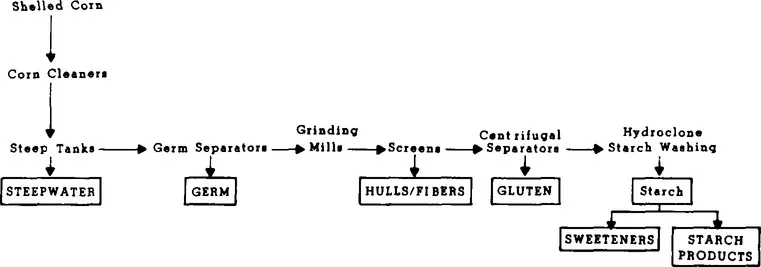
FIGURE 4. Production of corn syrups by a corn wet milling process.
(Adapted from Nutritive Sweetness from Corn, Corn Refiners Association, Inc., Washington, D.C., 1984.)
The discovery that starch was a polymer of glucose and could be transformed to a sweetener by heating with acid was an accident. G. S. C. Kirchoff, a chemist in a Russian ceramics laboratory, was looking for a substitute for gum arabic.13 Corn sugar was first produced commercially in 1866 in this country at a plant in Buffalo, N.Y. The process by which corn syrups are produced is shown in Figure 4. Corn is washed and then soaked or “steeped” in warm water containing sulfur dioxide. This softens and swells the corn, facilitating subsequent separations. The steeped corn is degerminated and then ground. The hulls and fibers are removed by passing the ground slurry through screens. Gluten is then separated by centrifugation. The remaining starch fraction is then washed, s...