
eBook - ePub
Anionic Surfactants
Analytical Chemistry, Second Edition,
John Cross
This is a test
- 368 pagine
- English
- ePUB (disponibile sull'app)
- Disponibile su iOS e Android
eBook - ePub
Anionic Surfactants
Analytical Chemistry, Second Edition,
John Cross
Dettagli del libro
Anteprima del libro
Indice dei contenuti
Citazioni
Informazioni sul libro
"Presents the most comprehensive coverage available of the detection, isolation, identification, and estimation of all anionic surfactants in a wide variety of samples in trace and macro quantities. Features new chapters on volumetric and trace analysis, molecular and mass spectroscopy, and chromatographic processes."
Domande frequenti
Come faccio ad annullare l'abbonamento?
È semplicissimo: basta accedere alla sezione Account nelle Impostazioni e cliccare su "Annulla abbonamento". Dopo la cancellazione, l'abbonamento rimarrà attivo per il periodo rimanente già pagato. Per maggiori informazioni, clicca qui
È possibile scaricare libri? Se sì, come?
Al momento è possibile scaricare tramite l'app tutti i nostri libri ePub mobile-friendly. Anche la maggior parte dei nostri PDF è scaricabile e stiamo lavorando per rendere disponibile quanto prima il download di tutti gli altri file. Per maggiori informazioni, clicca qui
Che differenza c'è tra i piani?
Entrambi i piani ti danno accesso illimitato alla libreria e a tutte le funzionalità di Perlego. Le uniche differenze sono il prezzo e il periodo di abbonamento: con il piano annuale risparmierai circa il 30% rispetto a 12 rate con quello mensile.
Cos'è Perlego?
Perlego è un servizio di abbonamento a testi accademici, che ti permette di accedere a un'intera libreria online a un prezzo inferiore rispetto a quello che pagheresti per acquistare un singolo libro al mese. Con oltre 1 milione di testi suddivisi in più di 1.000 categorie, troverai sicuramente ciò che fa per te! Per maggiori informazioni, clicca qui.
Perlego supporta la sintesi vocale?
Cerca l'icona Sintesi vocale nel prossimo libro che leggerai per verificare se è possibile riprodurre l'audio. Questo strumento permette di leggere il testo a voce alta, evidenziandolo man mano che la lettura procede. Puoi aumentare o diminuire la velocità della sintesi vocale, oppure sospendere la riproduzione. Per maggiori informazioni, clicca qui.
Anionic Surfactants è disponibile online in formato PDF/ePub?
Sì, puoi accedere a Anionic Surfactants di John Cross in formato PDF e/o ePub, così come ad altri libri molto apprezzati nelle sezioni relative a Physical Sciences e Physical & Theoretical Chemistry. Scopri oltre 1 milione di libri disponibili nel nostro catalogo.
Informazioni
1
Anionic Surfactants—An Introduction
JOHN CROSS Department of Physical Sciences, University of Southern Queensland, Toowoomba, Queensland, Australia
I. | An Introduction |
II. | Sources of the Hydrophobe A. Animal fats and vegetable oils B. Petrochemical sources |
III. | Common Types of Anionic Surfactants A. Alkylbenzenesulfonates B. Secondary alkanesulfonates C. Olefinsulfonates D. Ester sulfonates E. Other sulfonated surfactants F. Sulfate esters G. Carboxylates H. Phosphate esters I. Fluorinated surfactants |
IV. | Enter the Analyst A. The level of analysis B. Quality control C. Qualitative testing for anionic surfactants D. Isolation from the sample matrix |
V. | Some Important Readings A. General reading B. Analytical chemistry |
References |
I. AN INTRODUCTION
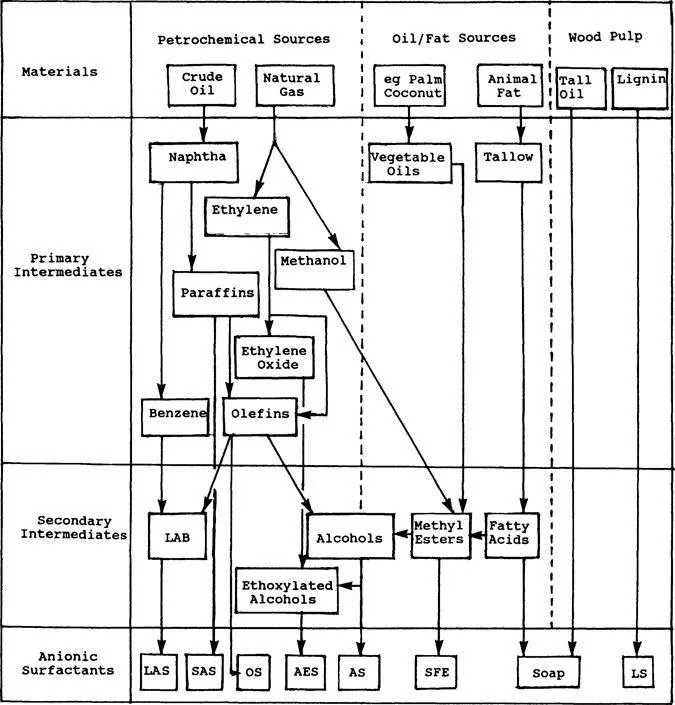
This chapter is intended to introduce the class of chemical compounds collectively known as anionic surfactants, the members of that class that have achieved major importance, and, as far as it affects the analyst, the raw materials, processes, and by-products that may be found. Some of the principal routes from the raw materials to surfactant are shown in Fig. 1. At this very early stage of this book, the author recommends to all readers interested in more detail that they consult the companion volume in the Surfactant Science Series, which concentrates on the preparation and properties of a wide range of anionic surfactants [1].
Surfactants are by necessity large molecules with molar masses usually in excess of 300. In the simplest case they consist of a nonpolar (usually hydrocarbon) chain attached to a highly polar or ionic group. These sections are known as the hydrophobe/oleophile/lipophile and the hydrophile/oleophobe/lipophobe, respectively, depending on the attraction—or lack thereof—to water or hydrocarbon oil.
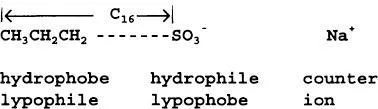
It is essential that the ends of the hydrophobe and the hydrophile are sufficiently remote from each other to react with surfaces and solvent molecules independently. The relative weightings of these groups was quantified by Griffin [2] in the form of the hydrophile-lipophile balance (HLB), a parameter that indicates the field of application to which the surfactant is most suited [3]. For example, cetyl alcohol (HLB = 1.0) relies upon a single -OH group to provide any hydrophilic character; it is virtually insoluble in water but will spread over the surface of water in a dam to provide a surface film that significantly reduces evaporation. Glyceryl monostearate possesses two -OH groups plus a less polar ester group and consequently has a higher HLB (3.8): such compounds are excellent water-in-oil emulsifiers. As the hydrophilic factor grows, the surfactant becomes more suited for use as a wetting agent (e.g., sorbitan monolaurate, HLB = 8.6) and an oil-in-water emulsifier (e.g., polyoxyethylene monostearate, HLB = 11-15). Anionic surfactants (anionics) have high HLBs due to the presence of ionic hydrophiles such as -COO– and -SO–3. HLBs range upwards from about 13 (e.g., sodium dodecanesulfonate, 13; sodium oleate, 18.5; and sodium dodecyl sulfate, 40): the major applications are to be found in the fields of detergency and solubilization.
Many treatises draw a distinction between “synthetic” and “natural” surfactants, the latter class being principally the soaps. Exactly what is natural about being boiled with sodium hydroxide is not made clear. As the public in general continues to strengthen its embrace of the concepts of “green” and “environmental capacity,” surfactant products have tended to become regarded as being “of natural origin” if they contain hydrophobes derived from fats and oils of recent animal or vegetable origin, despite the intensive chemical processing that follows. On the other hand, surfactants derived from fossil fuels are regarded as “synthetic” despite the fact that their ultimate origin was, in fact, vegetable/natural [4].
It is appropriate that the next section should examine the major sources of the hydrophobes, their treatment, and the likely structures that may arise as a consequence.
II. SOURCES OF THE HYDROPHOBE
The total global consumption of soaps is estimated to be some 8.5 million tons per annum (Mtpa). The fatty acids come almost exclusively from natural sources, i.e., animal fats and vegetable oils. Those derived from the oxidation of paraffin waxes tend to contain by-products and are of inferior quality. For the production of all other hydrophobes (except for the lignosulfonates), the fossil-based hydrophobes outweigh those from fats/oils sources 10-fold [5].
A. Animal Fats and Vegetable Oils
The major fatty acid content of some of the common oils and fats are listed in Table 1. The distribution of the various homologs and ratio of saturated-to-unsaturated acids was once believed to be a fingerprint of the parent oil, but in practice there is too much variation in composition arising from the particular strain of seedstock used and environmental factors such as soil type, nutrient availability, etc. In addition, a certain degree of fractionation, intended or otherwise, is likely to occur during processing.
Whether a particular triglyceride is a solid or liquid at ambient temperatures depends largely upon the length of the hydrocarbon chains (higher intermolecular forces) and the degree of unsaturation. The enforced planar arrangement around the ethylenic linkages causes a kink in what otherwise could become a regular conformation of the chains: this, in turn, prevents the chains from packing into a neat crystalline array. The unsaturated compounds, therefore, have a lower melting range than their saturated counterparts. Soft soaps, for these reasons, may be derived from oils with a high unsaturated fatty acid content, such as cottonseed oils: alternatively, the counterion used may be be potassium instead of the more usual sodium.
Tallow-sourced acids are frequently hydrogenated (i.e., hardened) and typically contain C14, C16, and C18 saturated acids with a ratio of 5:30:65 [7]. This process is so common that many marketing companies feel that it is unnecessary to declare that this has been carried out. Consequently, the term “tallow” attached to a product could refer to either the natural or the hydrogenated version.
The production of fats and oils is growing steadily and is expected to reach 100 Mtpa by the year 2000. Some 85% of oils are consumed as food, and the remainder, usually of poorer quality, is directed towards the oleochemicals industry. Fatty acids are liberated from the triglycerides by saponification, by direct high pressure hydrolysis with water, or by transesterification with methanol. The crude acids may be contaminated with mono- or diglycerides and various other components, which may induce color and odor. Fractionation by vacuum distillation of the free acids or distillation of the methyl esters will produce cuts from the homologous series according to variations in volatility: such processing will have little effect upon the ratio of saturated to unsaturated components.
TABLE 1 Typical Percent Fatty Acid Distribution Among Some Common Oils and Fats
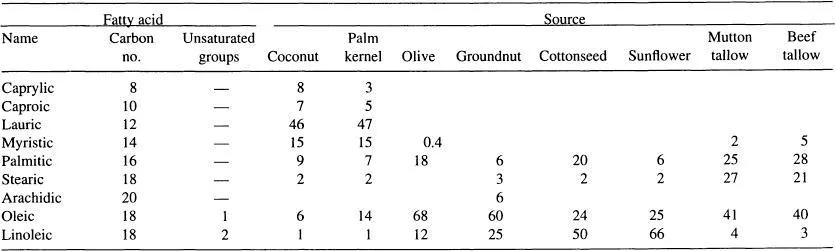
Source: Ref. 6.
For conversion to alcohol sulfates or alcohol ether sulfates, the methyl esters are catalytically hydrogenated to the intermediate fatty alcohols and methanol. In contrast to fatty alcohols produced from mineral sources, those from vegetable oils and animal fats will reliably be primary alcohols with linear chains. The most useful fatty alcohols from these sources are coconut alcohol (and different cuts thereof), tallow alcohol, cetyl/stearyl alcohol, oleyl alcohol, and oleyl/cetyl alcohol [8]. Another unique feature of hydrophobes derived from natural sources is the presence of only chains with even carbon numbers, e.g., C12, C14, C16, C18, etc.
Apart from natural fats and oils, the other major natural contributor to surfactant raw materials is the wood-pulping industry. By-products include (a) tall oil, a dark, oily mixture of fatty acids and rosin acids (basically polycyclic terpene carboxylic acids), which can be treated either by acid-washing or fractional distillation to yield fatty acids containing approximately 40% and 5% rosin acids, respectively, and (b) lignin, a polymeric material as complex as wood itself, which is separated from cellulose and other compounds. During the bisulfite bleaching process a significant amount of addition occurs to produce lignosulfonic acids with molecular masses of 100,000 or more [9].
B. Petrochemical Sources
The petrochemical industry provides the largest contribution to the hydrophobes of the nonsoapy anionic surfactants. The principal primary intermediates are benzene and linear paraffins, olefins, and alcohols. Hydrocarbon chains of suitable length must, in general, be generated either by polymerization of low molecular mass alkenes or by cracking of much larger molecules. Ethylene oxide and maleic anhydride (for sulfosuccinate synthesis) also arise from petroleum sources. In contrast to the products of fats/oils processing, petrochemically derived hydrocarbons contain both odd- and even-numbered carbon chains (unless produced by polymerization of ethylene).
1. Linear Paraffins
Linear paraffins in the molecular range C10–C18 are important as hydrophobes and are found in the kerosene and gas oil fractions. They are separated from other components of similar volatility by preferential adsorption onto synthetic zeolites, which function as molecular sieves: the small cross-sectional area of n- alkanes allows them to enter the cavities of the adsorbent, whereas the much bulkier branched-chain, cyclic and aromatic species are too large to do so. Paraffins in this range are used to produce alkanesulfates or are dehydrogenated to alkenes.
Higher molecular mass alkanes in the C20–C30 range are components of the lubricating oils fraction. They are not ...