
eBook - ePub
Nutrition
CHEMISTRY AND BIOLOGY, SECOND EDITION
Julian E. Spallholz, Mallory Boylan, Judy A. Driskell
This is a test
- 368 pagine
- English
- ePUB (disponibile sull'app)
- Disponibile su iOS e Android
eBook - ePub
Nutrition
CHEMISTRY AND BIOLOGY, SECOND EDITION
Julian E. Spallholz, Mallory Boylan, Judy A. Driskell
Dettagli del libro
Anteprima del libro
Indice dei contenuti
Citazioni
Informazioni sul libro
Category
NutritionSubcategory
Food ChemistryContact Editor: N. Frabotta
Domande frequenti
Come faccio ad annullare l'abbonamento?
È semplicissimo: basta accedere alla sezione Account nelle Impostazioni e cliccare su "Annulla abbonamento". Dopo la cancellazione, l'abbonamento rimarrà attivo per il periodo rimanente già pagato. Per maggiori informazioni, clicca qui
È possibile scaricare libri? Se sì, come?
Al momento è possibile scaricare tramite l'app tutti i nostri libri ePub mobile-friendly. Anche la maggior parte dei nostri PDF è scaricabile e stiamo lavorando per rendere disponibile quanto prima il download di tutti gli altri file. Per maggiori informazioni, clicca qui
Che differenza c'è tra i piani?
Entrambi i piani ti danno accesso illimitato alla libreria e a tutte le funzionalità di Perlego. Le uniche differenze sono il prezzo e il periodo di abbonamento: con il piano annuale risparmierai circa il 30% rispetto a 12 rate con quello mensile.
Cos'è Perlego?
Perlego è un servizio di abbonamento a testi accademici, che ti permette di accedere a un'intera libreria online a un prezzo inferiore rispetto a quello che pagheresti per acquistare un singolo libro al mese. Con oltre 1 milione di testi suddivisi in più di 1.000 categorie, troverai sicuramente ciò che fa per te! Per maggiori informazioni, clicca qui.
Perlego supporta la sintesi vocale?
Cerca l'icona Sintesi vocale nel prossimo libro che leggerai per verificare se è possibile riprodurre l'audio. Questo strumento permette di leggere il testo a voce alta, evidenziandolo man mano che la lettura procede. Puoi aumentare o diminuire la velocità della sintesi vocale, oppure sospendere la riproduzione. Per maggiori informazioni, clicca qui.
Nutrition è disponibile online in formato PDF/ePub?
Sì, puoi accedere a Nutrition di Julian E. Spallholz, Mallory Boylan, Judy A. Driskell in formato PDF e/o ePub, così come ad altri libri molto apprezzati nelle sezioni relative a Medicine e Biochemistry in Medicine. Scopri oltre 1 milione di libri disponibili nel nostro catalogo.
Informazioni
CHAPTER 1
The Elements of Life
PERIODIC ARRANGEMENT OF ELEMENTS AND THE ORGANIC CLUSTER
The periodic table (Figure 1.1) contains 111 elements. Only 90 of these elements occur naturally in the environment, and still fewer elements comprise the living world. Scientists have long sought means to predict, interpret, detect, and measure quantitatively the elements necessary for life. The discovery of life’s elements, contained by the totality of present knowledge, suggests that life has evolved from the less biologically complex to the more biologically complex. From bacteria to higher vertebrates and humans, nature has repeatedly selected for all life forms a basic group of only six elements. These six elements, the organic cluster (Figure 1.2), include the first element of the periodic table (see Figure 1.1), hydrogen, then carbon, nitrogen, oxygen, phosphorus, and sulfur. These six relatively small elements universally comprise most of the structural organization of the nutrients: proteins, carbohydrates, lipids, and vitamins. In addition, they make up most of the structural forms of the nucleic acids, deoxyribonucleic (DNA), and ribonucleic acids (RNA), and all the metabolic intermediates of metabolism.
The primordial selection of these six elements, which comprise the total organic bulk of all living matter, appears to have been made on the basis not only of physical size but also on chemical reactivity and the requirement to form intramolecular covalent bonds. Proteins, lipids, and carbohydrates are composed principally of monomers, small molecular units of carbon, oxygen, hydrogen, and nitrogen. The ability of carbon to form —C—C— bonds, extended carbon chains, and cyclic carbon compounds with an occasional mix of nitrogen and sulfur permitted the formation of the myriad of organic compounds. Silicon, located just below carbon in the periodic table, is also capable of forming extended chains. Chains of silicon, however, alternate with oxygen (—O—Si—O—Si—O), forming silicones. Such molecules were not biologically selected over carbon, for what would have been a much different type of macromolecular world.
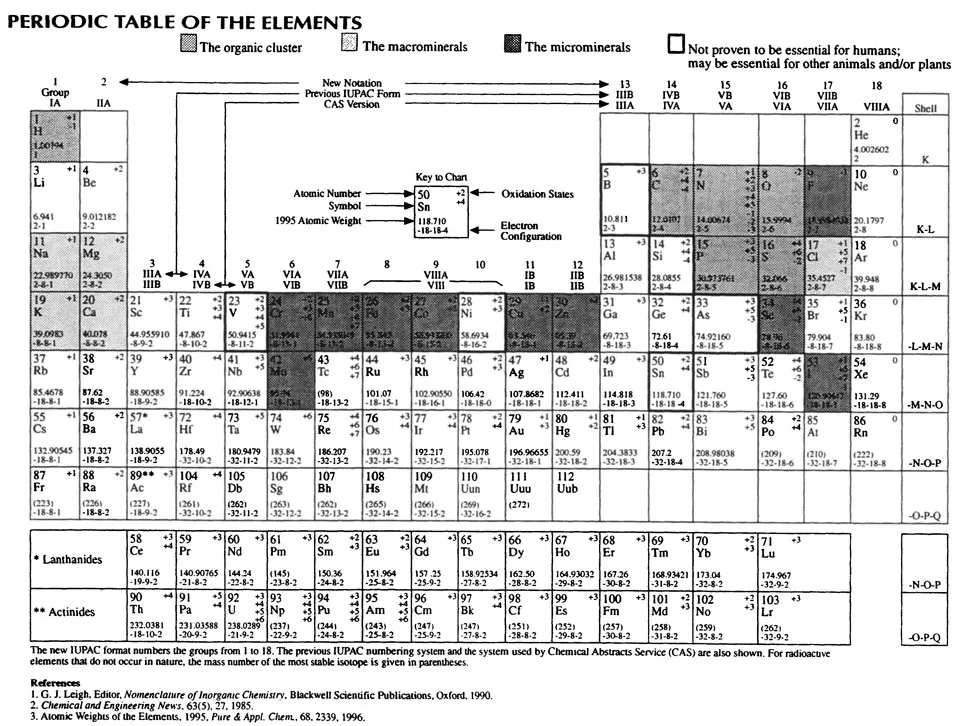
Figure 1.1 Periodic table of the elements.
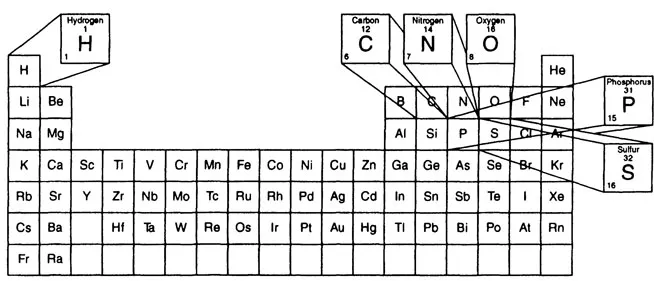
Figure 1.2 Organic cluster.
The elements of the organic cluster appear also to have been selected because of their abundance in the primordial atmosphere at the time the first molecules, probably amino acids, were found in the primordial soup. Evidence does suggest that the composition of the primordial atmosphere was probably much different from our present atmosphere in that it contained no oxygen but was comprised mostly of methane (CH4), ammonia (NH3), and smaller amounts of carbon monoxide (CO). Such gaseous mixtures saturated with water vapor, when subjected to electric discharge in the laboratory, result in the synthesis of small organic molecules resembling amino acids. The primordial atmosphere, as it is believed to have existed, together with the addition of sulfur as hydrogen sulfide (H2S) and driven by intense ultraviolet radiation through an ozone-free atmosphere, provided conditions suitable for the synthesis of the first biological organic molecules.
DISTRIBUTION OF ELEMENTS IN THE UNIVERSE, ON EARTH, AND IN THE HUMAN BODY
While the true origin of the organic molecules still remains open to conjecture, the association of the elemental composition of the human body to that of the universe, the primordial atmosphere, the earth’s crust, and seawater remains fascinating to contemplate. The universe is composed of approximately 91% hydrogen and 8.7% helium. All of the other 88 naturally occurring elements make up the remaining 0.3% of the universe and can be viewed within the periodic table as being derived from hydrogen and helium. There is within the periodic table a general inverse relationship between elemental abundance and atomic number. In different words, the larger the atomic number, generally, the rarer the element is in the universe, land, and sea. After H and He, we find four elements of the organic cluster, C, N, O, and S, to be relatively abundant in the universe. In the earth’s crust and atmosphere, we also find H, C, N, and O to be abundant. The composition of seawater closely approximates the elemental composition of the human body. People are composed of approximately 88.5% H and O. Salt water is 99% H and O, and only 1% of seawater includes all the other elements listed in Table 1.1.
Table 1.1 Approximate Elemental Composition (Percent Total Number Atoms)a
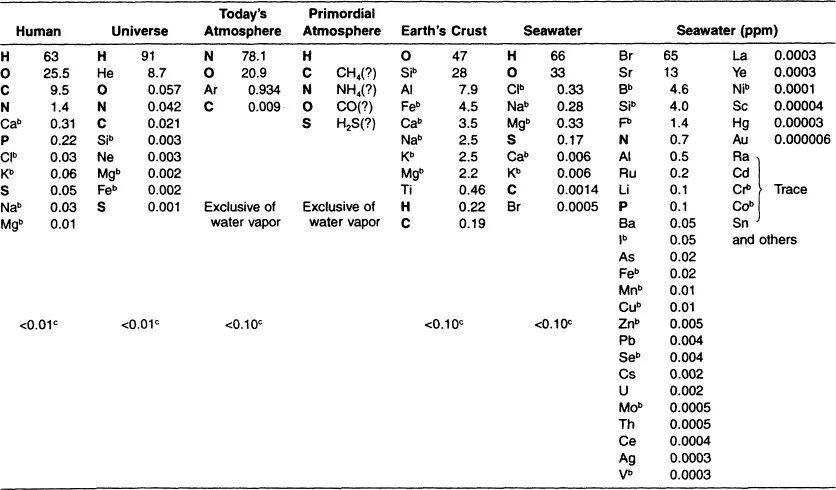
a Elements set in bold are of the organic cluster.
b Essential mineral.
c All other elements.
ELEMENTAL REQUIREMENTS OF MICROORGANISMS, PLANTS, ANIMALS, AND HUMANS
Bacteria, plants, animals, and humans require, in addition to the organic elements, various amounts and types of other elements, collectively referred to as minerals. Reexamination of Table 1.1 reveals that all major elements of the universe, with the exception of the inert gases helium (He) and neon (Ne), are included in plant and/or animal life. With the exception of Al, Ti, and Br, all major elements of the earth’s crust and seawater include those elements needed by bacteria, plants, animals, and humans.
As life forms increase in complexity from the simplest single cells of the amoebae and bacteria to the more complex plants and animals, there is a general increase in the number of required elements that comprise less than 0.1% and often less than 0.01% of our environment. The latter elements are the micro- and ultramicro- (trace) elements, elements required by life forms in very minute amounts. The elemental requirements of many bacteria, plants, and humans are given in Table 1.2. The required elements for bacteria, plants, and humans as representative of the vertebrates, shows 15 elements in common to all life forms. With few exceptions, bacteria and plants have similar known elemental requirements, with the elemental requirements of humans being the most extensive.
The biological complexity of life changes the requirements and the ways in which the need for the elements of the organic cluster (C, H, O, N, S, P) can be met. Bacterial requirements for the elements of the organic cluster are met by simple common salts, i.e., ammonium sulfate (NH4SO4) and an organic carbon source, i.e., glucose. Plants fulfill their need for the elements of the organic cluster from atmospheric CO2 and the remaining elements from soils (H2O, NH4SO4, NH4PO4). Animals and humans rely solely on the organic molecules — carbohydrates, lipids, proteins, and vitamins produced by bacteria and plant and animal foods — to fulfill their needs for the elements of the organic cluster. Macro-, micro-, and ultramicro- (trace) elements are provided to humans by foods from both plant and animal origin. A review of chemistry and biology concepts of importance in nutrition is given in Appendix A.
Table 1.2 Elements Required by Bacteria, Plants, and Humansa
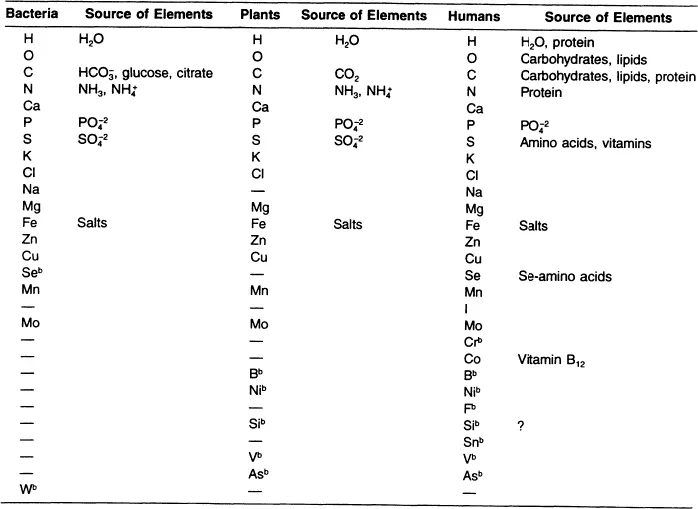
a Listed by decreasing amounts in humans. The requirements for elements by bacteria, plants, animals, and humans are similar, but the nutritional source of the elements becomes increasingly more complex for animals and humans than for bacteria and plants.
b May or may not have a biological function in all species within each class heading.
The Energy Nutrients
CHAPTER 2
Carbohydrates
The word carbohydrate has been compounded from the description of this group of organic molecules, the “carbon hydrates,” whose carbon compounds are extensively hydrated. The carbohydrates are either polyhydroxyaldehydes or polyhydroxyketones. Dietarily, carbohydrates provide approximately 50% of the caloric needs of Americans. Being derived from plants, vegetables, and cereal grains, a greater proportion of the caloric needs of people in developing countries are met by the carbohydrates.
MONOSACCHARIDES
Carbohydrates are grouped according to the number of carbon atoms per molecule, such as the trioses (three-carbon unit), the pentoses (five-carbon unit), and the hexoses (six-carbon unit). Nutritionally, the most important carbohydrates are the hexoses.
Glucose
The most important hexose metabolically is the monosaccharide, D-glucose. D-glucose is commonly referred to as blood glucose or blood sugar. Glucose comes in two epimeric forms, α-D-glucose and β-D-glucose (Figure 2.1). Glucose is found in each disaccharide as well as being found in all polysaccharides including glycogen, the major storage form of carbohydrate in the body. While glucose is the most important metabolic carbohydrate, glucose, galactose, fructose, and other monosaccharides are infrequent dietary carbohydrate constituents. Insignificant amounts of glucose are naturally consumed in diets.
Fructose and Galactose
The sweetest carbohydrate is fructose which is found in fruits, honey, and many syrups. Many people consume fructose which is added to carbonated beverages or products sweetened with high-fructose corn syrup. The third important monosaccharide is galactose, found as a constituent of lactose. Galactose, like glucose, is seldom found free in foods. In a rare c...