1.1 Introduction
The era of fluoropolymers began with a small mishap, which did not go unnoticed by the ingenious and observant Dr. Roy Plunkett of DuPont Company.[1] In 1938, he had been at DuPont for two years, concentrating mostly on the development of fluorinated refrigerants. He was experimenting with tetrafluoroethylene (TFE) for synthesis of a useful refrigerant (CClF2-CHF2).[2] The effort was spurred by the desire to create safe, nonflammable, nontoxic, colorless, and odorless refrigerants. On the morning of April 6, 1938, when Plunkett checked the pressure on a full cylinder of TFE, he found none. However, the cylinder had not lost weight. Careful removal of the valve and shaking the cylinder upside down yielded a few grams of a waxy looking white powder—the first polymer of tetrafluoroethylene.[2]
Plunkett analyzed the white powder, which was conclusively proven to be polytetrafluoroethylene (PTFE). The slippery PTFE could not be dissolved in any solvent, acid, or base and upon melting formed a stiff clear gel without flow.[3] Later, research led to the discovery of processing techniques similar to those used with metal powders. At the time, the Manhattan Project was seeking new corrosion-resistant material for gaskets, packings, and liners for UF6 handling. PTFE provided the answer and was used in production. The US government maintained a veil of secrecy over the PTFE project until well after the end of World War II.
Large-scale monomer synthesis and controlled polymerization were technical impediments to be resolved. Intensive studies solved these problems and small-scale production of Teflon® (trademark, 1944) began in 1947. In 1950, DuPont scaled up the commercial production of Teflon in the USA with the construction of a new plant in Parkersburg, West Virginia. In 1947, Imperial Chemical Industries built the first PTFE plant outside the US, in Western Europe. Since then, many more plants have been built around the globe. Over the last six decades, many forms of PTFE and copolymers of other monomers and TFE have been developed and commercialized.
The words of Plunkett himself best summarize the discovery of PTFE. He recounted the story of Teflon in a speech to the American Chemical Society at its April 1986 meeting in New York. “The discovery of polytetrafluoroethylene (PTFE) has been variously described as (i) an example of serendipity, (ii) a lucky accident and (iii) a flash of genius. Perhaps all three were involved. There is complete agreement, however, on the results of that discovery. It revolutionized the plastics industry and led to vigorous applications not otherwise possible.”[2]
1.2 What are Fluoropolymers?
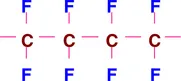
Traditionally, a fluoropolymer or fluoroplastic is defined as a polymer consisting of carbon (C) and fluorine (F). Sometimes these are referred to as perfluoropolymers to distinguish them from partially fluorinated polymers, fluoroelastomers and other polymers that contain fluorine in their chemical structure. For example fluorosilicone and fluoroacrylate polymers are not referred to as fluoropolymers. An example of a linear fluoropolymer is tetrafluoroethylene polymer (PTFE):
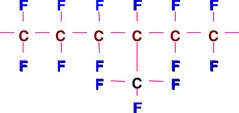
A simplistic analogy would be to the chemical composition of polyethylene [(-CH2-CH2-)n] where all the hydrogen atoms have been replaced by fluorine atoms. Of course, in practice PTFE and polyethylene are prepared in totally different ways. There are branched fluoropolymers such as fluorinated ethylene propylene polymer (FEP):
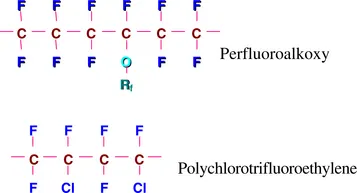
Oxygen (O) and chlorine (Cl) are present in the chemical structure of some commercial fluoropolymers. Examples include perfluoroalkoxy and polychlorotrifluoroethylene:
Rf is usually a perfluorinated group consisting of carbon and fluorine. Introduction of nonlinearity, oxygen and side chains, or chlorine invoke a variety of polymer properties which will be dealt with later in this book.
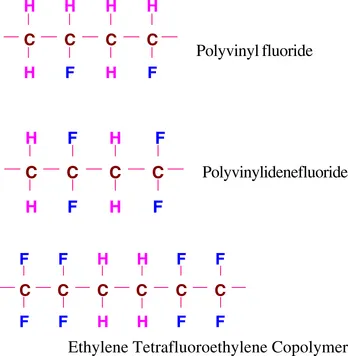
There is a second class of fluoropolymers called “partially fluorinated” in contrast to “perfluorinated polymers.” These molecules include hydrogen (H) in addition to fluorine and carbon. Examples include polyvinylfluoride, polyvinylidenefluoride, and ethylene tetrafluoroethylene copolymer.
Partially fluorinated fluoropolymers are significantly different from the perfluoropolymers with respect to properties and processing characteristics. For example, per...