
eBook - ePub
Progress in Nuclear Physics
The Leading International Review Series in Nuclear Physics, Vol. 6
O. R. Frisch, O. R. Frisch
This is a test
Condividi libro
- 306 pagine
- English
- ePUB (disponibile sull'app)
- Disponibile su iOS e Android
eBook - ePub
Progress in Nuclear Physics
The Leading International Review Series in Nuclear Physics, Vol. 6
O. R. Frisch, O. R. Frisch
Dettagli del libro
Anteprima del libro
Indice dei contenuti
Citazioni
Informazioni sul libro
Progress in Nuclear Physics: Volume 6 is a collection of scientific papers in the field of experimental and theoretical physics. The compendium contains research papers covering a wide and diverse range of subjects in various areas of physics. The book provides contributions that discuss the methods for measuring atomic masses; the preparation of pure or enriched isotopes through electromagnetic separators; the study of nuclear moments; the spectroscopy of mesonic atoms; and parity nonconservation in weak interactions. Theoretical and experimental physicists will find this book very insightful.
Domande frequenti
Come faccio ad annullare l'abbonamento?
È semplicissimo: basta accedere alla sezione Account nelle Impostazioni e cliccare su "Annulla abbonamento". Dopo la cancellazione, l'abbonamento rimarrà attivo per il periodo rimanente già pagato. Per maggiori informazioni, clicca qui
È possibile scaricare libri? Se sì, come?
Al momento è possibile scaricare tramite l'app tutti i nostri libri ePub mobile-friendly. Anche la maggior parte dei nostri PDF è scaricabile e stiamo lavorando per rendere disponibile quanto prima il download di tutti gli altri file. Per maggiori informazioni, clicca qui
Che differenza c'è tra i piani?
Entrambi i piani ti danno accesso illimitato alla libreria e a tutte le funzionalità di Perlego. Le uniche differenze sono il prezzo e il periodo di abbonamento: con il piano annuale risparmierai circa il 30% rispetto a 12 rate con quello mensile.
Cos'è Perlego?
Perlego è un servizio di abbonamento a testi accademici, che ti permette di accedere a un'intera libreria online a un prezzo inferiore rispetto a quello che pagheresti per acquistare un singolo libro al mese. Con oltre 1 milione di testi suddivisi in più di 1.000 categorie, troverai sicuramente ciò che fa per te! Per maggiori informazioni, clicca qui.
Perlego supporta la sintesi vocale?
Cerca l'icona Sintesi vocale nel prossimo libro che leggerai per verificare se è possibile riprodurre l'audio. Questo strumento permette di leggere il testo a voce alta, evidenziandolo man mano che la lettura procede. Puoi aumentare o diminuire la velocità della sintesi vocale, oppure sospendere la riproduzione. Per maggiori informazioni, clicca qui.
Progress in Nuclear Physics è disponibile online in formato PDF/ePub?
Sì, puoi accedere a Progress in Nuclear Physics di O. R. Frisch, O. R. Frisch in formato PDF e/o ePub, così come ad altri libri molto apprezzati nelle sezioni relative a Physical Sciences e Quantum Theory. Scopri oltre 1 milione di libri disponibili nel nostro catalogo.
Informazioni
Argomento
Physical SciencesCategoria
Quantum Theory1
ISOTOPE SEPARATION BY MULTISTAGE METHODS
T.F. Johns
Publisher Summary
Isotopes of an element can be separated either by single-stage or multistage processes. This chapter describes the multistage methods for isotope separation. The single-stage method of importance is the electro-magnetic method. The electromagnetic method enables large enrichments to be achieved in a single stage and it is versatile because the same machine can be used to separate the isotopes of any element. The other methods of separation depend on the fact that there are slight differences in the properties of isotopic substances, as a result of which slight separations of isotopes occur in a variety of simple chemical and physical processes such as distillation or diffusion. The important multistage methods of separation are electrolysis, fractional distillation, chemical exchange methods, and gaseous and thermal diffusion, though many other methods have been used to produce partial separation of isotopes.
CONTENTS
1 INTRODUCTION
2 METHODS EMPLOYED
2.1 Electrolytic methods
2.2 Distillation
2.3 Chemical exchange methods
2.4 Separation by diffusion
2.5 Thermal diffusion
2.6 Other methods of separation
2.7 Laboratory-scale separation of isotopes
3 METHODS OF ISOTOPIC ANALYSIS
4 GENERAL REMARKS ON THE DESIGN OF ISOTOPE-SEPARATION PLANTS
5 COMPARISON OF THE DIFFERENT SEPARATION METHODS
6 ISOTOPES WHICH HAVE BEEN SEPARATED
7 SEPARATION OF OTHER ISOTOPES
REFERENCES
1 INTRODUCTION
ISOTOPES of an element can be separated either by single-stage or multistage processes. The only single-stage method of importance is the electro-magnetic method, which is described in detail in an adjoining article (see p. 162). The other methods of separation depend on the fact that there are very slight differences in the properties of isotopic substances, as a result of which very slight separations of isotopes occur in a variety of simple chemical and physical processes such as distillation or diffusion. When such processes are repeated many times (usually several hundred times) useful separations can be obtained. The most important multistage methods of separation are electrolysis, fractional distillation, chemical exchange methods, and gaseous and thermal diffusion, though many other methods have been used to produce at least partial separation of isotopes.
The electromagnetic method is attractive for several reasons; it enables large enrichments to be achieved in a single stage; it is versatile in that the same machine can be used to separate the isotopes of any element; all the isotopes can be collected simultaneously, and it is as easy to collect the isotopes of intermediate mass as the extreme ones. Electromagnetic separation is, however, a very expensive process, so that although its versatility justifies the operational costs for separating small quantities of a wide variety of isotopes, the process becomes prohibitively expensive for large quantities, say in excess of 10-100 grams depending on the element, the limit being even lower in the case of rare isotopes. In fact the only large-scale plant of this type, used for separating kilogram quantities of 235U (incidentally a heavy element for which this process is economically more favourable) in 1942–4, was later closed down and superseded by the more economical gaseous-diffusion plant.
When quantities of more than a few grams of separated isotopes are required, it is normally desirable to use multistage methods of separation. The more important of these are described in this review. They are usually, though not always, specific to the isotopes of one particular element. However, the cost of production is usually so much less than the cost of electromagnetic separation that the additional capital cost of research and equipment is more than justified.
Since this review is intended primarily for nuclear physicists, and not for specialists in the field of isotope separation, it contains only a rather general account of some of the more important separation methods, and no attempt has been made to discuss the basic principles involved in the separation processes. An attempt has been made, however, to indicate the relative advantages and limitations of the various separation methods. Some general features of cascade operation are given in the discussion of the electrolytic method (which is for this reason discussed rather fully), and of continuous countercurrent separation methods in discussing fractional distillation. A number of features common to nearly all isotope separation plants are discussed in paragraph 4. No attempt has been made to give comprehensive references, but some of the more important and representative papers have been fisted.
2 METHODS EMPLOYED
2.1 Electrolytic methods
A slight separation of isotopes can be obtained by electrolysis, the isotopic abundance in the products of the electrolysis differing from the abundance in the parent material. The electrolytic method of separation is not of much practical importance except in the case of deuterium, but it is of interest because it was the first method to be used for the separation of really large quantities of an isotope. The Norwegian heavy-water plant (TRONSTAD, 1934) was producing several tons of heavy water per annum before 1939.
When water is electrolysed, the gas evolved at the cathode contains a lower proportion of deuterium than the original water. If c is the concentration of deuterium in the electrolytic gas, (1 – c) the concentration of hydrogen, and C,(1 – C) are the corresponding concentrations in the water, then
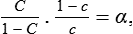
where α is known as the (single-stage) separation factor for this process, and is, in this case, about 6 (see Table 1).
Table 1
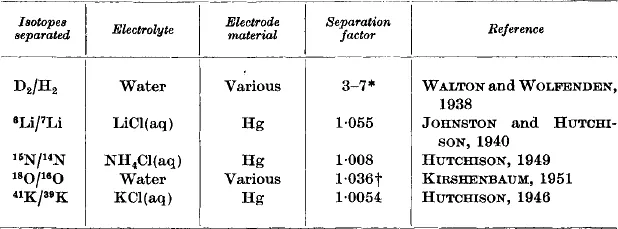
*Depends on the electrode material and current density, and to some extent on the nature of the electrolyte. For nickel cathodes, separation factors of 6–7 are normally obtained.
†Figures near 1.01 have also been quoted, but 1.036 appears to be a more probable figure.
If a large quantity of water, volume V0, is electrolysed until only a (small) volume V remains, then the concentration of...