
eBook - ePub
Imaging from Cells to Animals In Vivo
Margarida Barroso, Xavier Intes, Margarida Barroso, Xavier Intes
This is a test
Condividi libro
- 352 pagine
- English
- ePUB (disponibile sull'app)
- Disponibile su iOS e Android
eBook - ePub
Imaging from Cells to Animals In Vivo
Margarida Barroso, Xavier Intes, Margarida Barroso, Xavier Intes
Dettagli del libro
Anteprima del libro
Indice dei contenuti
Citazioni
Informazioni sul libro
Imaging from Cells to Animals In Vivo offers an overview of optical imaging techniques developed over the past two decades to investigate biological processes in live cells and tissues. It comprehensively covers the main imaging approaches used as well as the application of those techniques to biological investigations in preclinical models. Among the areas covered are cell metabolism, receptor-ligand interactions, membrane trafficking, cell signaling, cell migration, cell adhesion, cytoskeleton and other processes using various molecular optical imaging techniques in living organisms, such as mice and zebrafish.
Features
- Brings together biology and advanced optical imaging techniques to provide an overview of progress and modern methods from microscopy to whole body imaging.
- Fills the need for a comprehensive view of application-driven development and use of new tools to ask new biological questions in the context of a living system.
- Includes basic chapters on key methods and instrumentation, from fluorescence microscopy and imaging to endoscopy, optical coherence tomography and super-resolution imaging.
- Discusses approaches at different length scales and biomedical applications to the study of single cell, whole organ, and whole organism behavior.
- Addresses the impact on discovery, such as cellular function as implicated in human disease and translational medicine, for example in cancer diagnosis.
Domande frequenti
Come faccio ad annullare l'abbonamento?
È semplicissimo: basta accedere alla sezione Account nelle Impostazioni e cliccare su "Annulla abbonamento". Dopo la cancellazione, l'abbonamento rimarrà attivo per il periodo rimanente già pagato. Per maggiori informazioni, clicca qui
È possibile scaricare libri? Se sì, come?
Al momento è possibile scaricare tramite l'app tutti i nostri libri ePub mobile-friendly. Anche la maggior parte dei nostri PDF è scaricabile e stiamo lavorando per rendere disponibile quanto prima il download di tutti gli altri file. Per maggiori informazioni, clicca qui
Che differenza c'è tra i piani?
Entrambi i piani ti danno accesso illimitato alla libreria e a tutte le funzionalità di Perlego. Le uniche differenze sono il prezzo e il periodo di abbonamento: con il piano annuale risparmierai circa il 30% rispetto a 12 rate con quello mensile.
Cos'è Perlego?
Perlego è un servizio di abbonamento a testi accademici, che ti permette di accedere a un'intera libreria online a un prezzo inferiore rispetto a quello che pagheresti per acquistare un singolo libro al mese. Con oltre 1 milione di testi suddivisi in più di 1.000 categorie, troverai sicuramente ciò che fa per te! Per maggiori informazioni, clicca qui.
Perlego supporta la sintesi vocale?
Cerca l'icona Sintesi vocale nel prossimo libro che leggerai per verificare se è possibile riprodurre l'audio. Questo strumento permette di leggere il testo a voce alta, evidenziandolo man mano che la lettura procede. Puoi aumentare o diminuire la velocità della sintesi vocale, oppure sospendere la riproduzione. Per maggiori informazioni, clicca qui.
Imaging from Cells to Animals In Vivo è disponibile online in formato PDF/ePub?
Sì, puoi accedere a Imaging from Cells to Animals In Vivo di Margarida Barroso, Xavier Intes, Margarida Barroso, Xavier Intes in formato PDF e/o ePub, così come ad altri libri molto apprezzati nelle sezioni relative a Biowissenschaften e Biophysik. Scopri oltre 1 milione di libri disponibili nel nostro catalogo.
Informazioni
Section I
Overview of Imaging Methods and Instrumentation
1
Fluorescence Microscopy Techniques
Mehmet S. Ozturk and Robert Prevedel
CONTENTS
1.1 Introduction
1.2 Principles of Fluorescence Imaging
1.2.1 Photophysics of Fluorescence
1.2.2 Effects of the Host Environment on Fluorescent Molecules
1.2.3 Effects of Fluorescent Molecules on Host Environment
1.2.4 Fluorophore Types
1.2.5 Endogenous Fluorophores (EnF)
1.2.6 Exogenous Fluorophores (ExF)
1.2.6.1 Fluorescent Dyes
1.2.6.2 Quantum Dots
1.2.6.3 Fluorescence Indicators
1.3 Principles of Microscopy
1.3.1 Image Formation and Magnification
1.3.2 Numerical Aperture and Spatial Resolution
1.3.3 Fluorescence Microscopy Techniques for In Vivo Studies
1.3.3.1 Deconvolution
1.3.3.2 Confocal Microscopy
1.3.3.3 Light Sheet Microscopy
1.3.3.4 Multiphoton Microscopy
1.4 Summary
References
1.1 Introduction
Fluorescence microscopy has been a quintessential and enabling tool for life scientists for more than a century and, as such, a source of major discoveries in biology. These days, advanced imaging techniques based on fluorescence dyes and proteins are routinely used to visualize subtle processes in living cells at a subcellular resolution and often with molecular specificity. These microscopes have changed our visual perception as well as our understanding of cellular mechanisms. They allow us to learn from direct observation and thereby come to a mechanistic understanding of how complex, multicellular living systems operate. In this chapter, we will introduce the reader to the basic principles in fluorescence microscopy, starting with the photophysics of fluorescence molecules. Here, we are purposely focusing on fluorescent markers suitable for in vivo imaging and discussing their relevant parameters, which influence their function and respective applications. The remainder of this chapter, then, is dedicated to a technical discussion and review of various fluorescence microscopy techniques that are relevant for in vivo studies.
1.2 Principles of Fluorescence Imaging
1.2.1 Photophysics of Fluorescence
A fluorescence molecule has to fulfill many requirements in order to be most useful for a particular in vivo study. Thus, it is helpful to understand the basic principles underlying fluorescence signal generation. In this section, we will introduce the mechanism and parameters relevant for fluorescent imaging. While today there exists an extensive library of available fluorophores with well-documented parameters, environmental conditions can often affect those parameters positively or negatively. Therefore, we will also briefly discuss and, where possible, quantify these effects.
Inherent Parameters of Fluorophores
Quantum Electronic Behavior: Fluorescence is an incoherent light emission phenomenon that stems from the quantum mechanical behavior of electrons within a molecule. Even in the case of a coherent (light) source excitation, the resulting emission will be incoherent, i.e. not in phase with the excitation light, because of the vibrational relaxation within the molecule (Wang, Lihong, and Wu, 2007).
The underlying mechanism works as follows. An incoming (excitation) photon is absorbed by the molecule, residing in the ground state (S 0). Thus, one of the electrons makes a transition to an excited state (S 0–S 1). The excited electron dwells in the excited state for an average time (fluorescence lifetime), during which it experiences a series of nonradiative transitions (e.g. internal conversion, intersystem crossing, etc.), before spontaneously returning to the S 0 state (S 1–S 0) by radiative transition. The energy difference between the excited and ground state is radiated in the form of visible/near infrared (VIS/NIR) light (Figure 1.1).
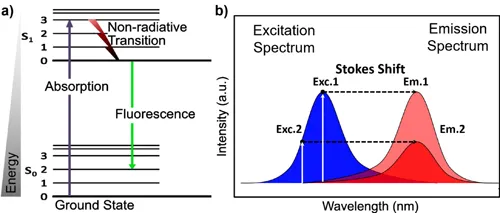
Because some of the energy is effectively lost to vibrational relaxation of the molecule, the fluorescence emission results in less energetic, i.e. longer wavelength (red-shifted), photons. This energy difference is called Stokes shift (Figure 1.1b). Note that the excitation and emission spectra often display some symmetry. This “mirror spectrum” is due to the similarity of vibrational levels for the ground state (S 0) and the excited state (S 1) (Sauer, Hofkens, and Enderlein, 2011).
The excitation rate (k ex), i.e. the rate at which electrons make a transition from the ground state to the excited state, is directly proportional to the excitation light intensity (I ex) and the molecular absorption cross section (σ), k ex = I exσ. To illustrate the fluorescence working principle quantitatively, let us assume a generic fluorophore with a molecular cross section (σ) of 3 × 10−16 cm2. If our molecule is illuminated by a 1mW light source (560 nm) with a beam radius of 0.5 µm, the power density will be ~1.3 × 105 W/cm2, which corresponds to 3.1 × 1023 photons/cm2sec (according to ). Therefore, the excitation rate of a single molecule within the focus will be k ex = 9.58 × 107 photons/sec. All the excited electrons will experience a de-excitation at a rate of k dex, which is the sum of radiative (k r) and nonradiative (k nr) de-excitation processes, k dex = k r + k nr. Next, we establish a relationship between the fraction of excited state electrons (x) and de-excited electrons (1–x), which is (e.g. x = 0.75) (Panula, 2003). Then, k dex becomes 3.2 × 107 photons/sec. The aim is here to identify the radiative relaxation rate (k r), for which we need to know the so-called quantum yield (Φ). The quantum yield is the ratio of electrons that undergo radiative relaxation (k r) compared to all excited electrons undergoing both radiative and nonradiative de-excitation processes (k r + k nr),
For simplicity, let us assume a quantum yield (Φ) of 1/3, which results in a radiative relaxation rate k r of 1,07 × 107 photons/sec, approximately.
The expected fluorescence intensity (I f) of a single molecule at the detector further depends on the overall efficiency of the imaging system, η (a composite metric that includes light collection efficiency of optics and the quantum efficiency of the detector), or I f = k dexΦη. A realistic value for η is ~10%, which leaves us with 1.07 × 106 photon/sec of fluorescence from a molecule. Typical exposure times of detectors span from µsec for a confocal or multiphoton microscope to msec for camera-based microscopes such as a wide-field or light sheet; therefore, this fluorescence yield will result in between 1 photon (exposure time 1 µsec) to 103 photons (exposure time 1 ms). Eventually, the total number of photons will depend on the number of molecules that are simultaneously excited by the focused light. For our illustration purpose, let us assume a fluorophore concentration of 1 µM, which translates to ~2,520 molecules inside a spherical focal volume of 1 µm radius (M = N × n/V), where M is the molar concentration, N is the Avogadro’s Number (6.02 × 1023), n is the amount of mol, and V is the volume (1L = 1015 µL). Thus, we end up with a range of 103–106 photons per detector pixel in our example calculation, which also resembles typical values for bright fluorescence sam...