Water Resources
An Integrated Approach
Joseph Holden, Joseph Holden
- 468 pagine
- English
- ePUB (disponibile sull'app)
- Disponibile su iOS e Android
Water Resources
An Integrated Approach
Joseph Holden, Joseph Holden
Informazioni sul libro
Now in its second edition, Water Resources: An Integrated Approach provides students with a comprehensive overview of natural processes associated with water and the modifications of these processes by humans through climate change and land management, water-related health issues, engineering approaches to water and socio-economic processes of huge importance to water resources. The book contains chapters written by 24 specialist contributors, providing expert depth of coverage to topics.
The text introduces the basic properties of water and its importance to society and the nature of the different regional imbalances between water resource availability and demand. It guides the reader through the changing water cycle impacted by climate and land management, water flows in river basins, surface water quality, groundwater and aquatic ecosystems, and covers the role of water in human health and associated hazards before turning to engineering solutions to water and wastewater treatment and reuse. The book deals with physical and social management strategies required for water resource planning, the economics of water and treatment of issues associated with conflict over water. The concept of virtual water is covered before the text concludes with a chapter considering the challenges of predicting future water issues in a rapidly changing world and where environmental systems can behave in a non-linear way. The need to work across disciplines to address challenges that are connected at both local and global scales is highlighted. Water Resources also includes global examples from both the developing and developed world. There are 58 case study boxes. Each chapter is supplemented with these case studies and with reflective questions, project ideas and further reading, as well as links to a glossary of terms. The book is richly illustrated throughout with over 160 full-colour diagrams and photographs.
The text provides a novel interdisciplinary approach to water in a changing world, from an environmental change perspective and interrelated social, political and economic dimensions. It will be an indispensable guide to undergraduates studying water resources and management, geography of water, and water in the environment.
Domande frequenti
Informazioni
CHAPTER ONE
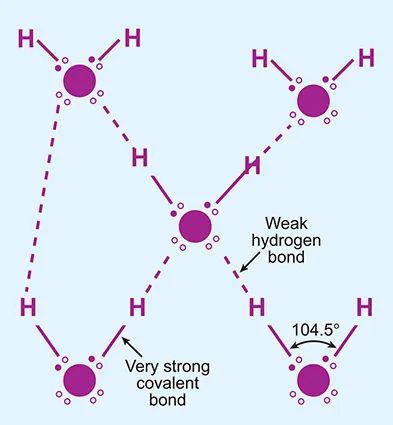
Water basics
- describe the nature of water;
- describe some of the roles of water in historic and contemporary society;
- outline some of the major global water resource issues.
1.1 Introduction
1.2 What is water?