
eBook - ePub
Solid State Chemistry
An Introduction
Elaine A. Moore, Lesley E. Smart
This is a test
Condividi libro
- 442 pagine
- English
- ePUB (disponibile sull'app)
- Disponibile su iOS e Android
eBook - ePub
Solid State Chemistry
An Introduction
Elaine A. Moore, Lesley E. Smart
Dettagli del libro
Anteprima del libro
Indice dei contenuti
Citazioni
Informazioni sul libro
Solid State Chemistry: An Introduction presents a wide range of the synthetic and physical techniques used to prepare and characterize solids. Going beyond this, this largely nonmathematical introduction to solid state chemistry includes the bonding and electronic, magnetic, electrical and optical properties of solids. Solids of particular interest – porous solids, superconductors and nanostructures are included. Practical examples of applications and modern developments are given. It offers students the opportunity to apply their knowledge in real-life situations and serve them well throughout their degree course.
Domande frequenti
Come faccio ad annullare l'abbonamento?
È semplicissimo: basta accedere alla sezione Account nelle Impostazioni e cliccare su "Annulla abbonamento". Dopo la cancellazione, l'abbonamento rimarrà attivo per il periodo rimanente già pagato. Per maggiori informazioni, clicca qui
È possibile scaricare libri? Se sì, come?
Al momento è possibile scaricare tramite l'app tutti i nostri libri ePub mobile-friendly. Anche la maggior parte dei nostri PDF è scaricabile e stiamo lavorando per rendere disponibile quanto prima il download di tutti gli altri file. Per maggiori informazioni, clicca qui
Che differenza c'è tra i piani?
Entrambi i piani ti danno accesso illimitato alla libreria e a tutte le funzionalità di Perlego. Le uniche differenze sono il prezzo e il periodo di abbonamento: con il piano annuale risparmierai circa il 30% rispetto a 12 rate con quello mensile.
Cos'è Perlego?
Perlego è un servizio di abbonamento a testi accademici, che ti permette di accedere a un'intera libreria online a un prezzo inferiore rispetto a quello che pagheresti per acquistare un singolo libro al mese. Con oltre 1 milione di testi suddivisi in più di 1.000 categorie, troverai sicuramente ciò che fa per te! Per maggiori informazioni, clicca qui.
Perlego supporta la sintesi vocale?
Cerca l'icona Sintesi vocale nel prossimo libro che leggerai per verificare se è possibile riprodurre l'audio. Questo strumento permette di leggere il testo a voce alta, evidenziandolo man mano che la lettura procede. Puoi aumentare o diminuire la velocità della sintesi vocale, oppure sospendere la riproduzione. Per maggiori informazioni, clicca qui.
Solid State Chemistry è disponibile online in formato PDF/ePub?
Sì, puoi accedere a Solid State Chemistry di Elaine A. Moore, Lesley E. Smart in formato PDF e/o ePub, così come ad altri libri molto apprezzati nelle sezioni relative a Sciences physiques e Chimie physique et théorique. Scopri oltre 1 milione di libri disponibili nel nostro catalogo.
Informazioni
1 An Introduction to Crystal Structures
The last few decades have seen much exciting research into solid-state chemistry. We have seen immense strides in the development of nanotechnology and solids contributing to the development of sustainable energy devices such as photovoltaic cells, fuel cells, and batteries, to mention but a few areas. It would be impossible to cover all the developments and topics in detail in an introductory text such as this, but we endeavour to give you a flavour of the excitement that some of the research has engendered and perhaps, more importantly, sufficient background with which to understand these developments and those that are yet to come.
Most substances, helium being a notable exception, if cooled sufficiently form a solid phase; the vast majority form one or more crystalline phases, where the atoms, molecules, or ions pack together to form a regular repeating array. This book is concerned mostly with the structures of metals, ionic solids, and extended covalent structures; structures that do not contain discrete molecules as such, but that comprise extended arrays of atoms or ions. We look at the structure of, and bonding in, these solids, how the properties of a solid depend on its structure, and how the properties can be modified by changes to the structure.
1.1 Introduction
To understand the solid state, we need to have some insight into the structure of simple crystals and the forces that hold them together, so it is here that we start this book. Crystal structures are usually determined by the technique of X-ray crystallography. This technique relies on the fact that the distances between the atoms in the crystals are of the same order of magnitude as the wavelength of X-rays (of the order of 1 Å or 100 pm): a crystal thus acts as a three-dimensional diffraction grating to a beam of X-rays. The resulting diffraction pattern can be interpreted to give the internal positions of the atoms in the crystal very precisely, thus defining interatomic distances and angles. (Some of the principles underlying this technique are discussed in Chapter 2, where we review the physical methods available for characterising solids.) Most of the structures discussed in this book would have been determined in this way.
1.2 Lattices and Unit Cells
Crystals are regular-shaped solid particles with flat shiny faces. In 1664, Robert Hooke first noted that the regularity of their external appearance is a reflection of a high degree of internal order. Crystals of the same substance, however, vary in shape considerably. In 1671, Nicolas Steno observed that this is not because their internal structure varies, but because some faces develop more than others. The angles between similar faces on different crystals of the same substance are always identical. The constancy of the interfacial angles reflects the internal order within the crystals. Each crystal is derived from a basic ‘building block’ that repeats over and over again, in all directions, in a perfectly regular way. This building block is known as the unit cell.
In order to talk about and compare the many thousands of crystal structures that are known, there has to be a way of defining and categorising the structures. This is achieved by defining the shape, symmetry, and also the size of each unit cell and the positions of the atoms within it.
1.2.1 Lattices
The simplest regular array is a line of evenly spaced objects such as that depicted by the commas in Figure 1.1a. There is a dot at the same place in each object: if we now remove the objects leaving the dots, we have a line of equally spaced dots of spacing a (Figure 1.1b). The line of dots is called the lattice, and by definition each lattice point (dot) has identical surroundings. This is the only example of a one-dimensional lattice and it can vary only in the spacing a. There are five possible two-dimensional lattices, and everyday examples of these can be seen all around in wallpapers and tiling.
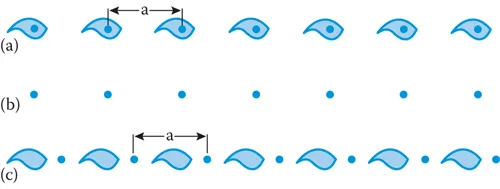
1.2.2 One- and Two-Dimensional Unit Cells
The unit cell for the one-dimensional lattice in Figure 1.1a lies between the two vertical lines. If we took this unit cell and repeated it over and over again, we would reproduce the original array. Notice that it does not matter where in the structure we place the lattice points as long as they each have identical surroundings. In Figure 1.1c, we have moved th...