![]()
Technological Advances
Hiort O, Ahmed SF (eds): Understanding Differences and Disorders of Sex Development (DSD).
Endocr Dev. Basel, Karger 2014, vol 27, pp 41-52 (DOI: 10.1159/000363612)
______________________
Steroid Biochemistry
Clemens Kamratha · Stefan A. Wudya · Nils Kroneb
aDivision of Pediatric Endocrinology and Diabetology, Laboratory for Translational Hormone Analytics in Pediatric Endocrinology, Steroid Research and Mass Spectrometry Unit, Centre of Child and Adolescent Medicine, Justus Liebig University, Giessen, Germany; bCentre for Endocrinology, Diabetes and Metabolism, School of Clinical and Experimental Medicine, University of Birmingham, Birmingham, UK
______________________
Abstract
Accurate analysis of steroid hormones represents an essential part in the evaluation of a patient with disorders or differences in sex development. Analytical methods based on mass spectrometry (MS) have become the state-of-the-art methodology allowing for the most specific qualitative and quantitative determination of steroid hormones and their metabolites. Liquid chromatography linked with tandem MS (LC-MS/MS) allows for rapid as well as highly specific and sensitive targeted steroid hormone analysis of multiple analytes from a single sample. Urinary steroid profile analysis by gas chromatography (GC)-MS is a non-invasive diagnostic approach and provides qualitative and quantitative data on the global excretion of steroid hormone metabolites. GC-MS remains the most powerful discovery tool for defining inborn errors of steroidogenesis, whereas LC-MS/MS represents a highly sensitive and specific method for targeted steroid hormone analysis.
© 2014 S. Karger AG, Basel
Steroidogenesis
The majority of steroidogenic enzymes are cytochrome P450 (CYP) enzymes catalysing redox reactions. These biochemical conversions crucially rely on electron supply via specific electron transfer chains. CYP type 1 enzymes are localised to the mitochondrion and receive their electrons via adrenodoxin reductase and adrenodoxin. P450 side-chain cleavage enzyme (CYP11 A1), 11β-hydroxylase (CYP11B1) and aldo-sterone synthase (CYP11B2) are all CYP type 1 enzymes. In contrast, 17α-hydroxylase (CYP17A1), 21-hydroxylase (CYP21A2) and aromatase (CYP19A1) are CYP type 2 enzymes localised to the endoplasmic reticulum. These microsomal CYP enzymes rely on electron transfer from P450 oxidoreductase (POR) to facilitate the hydroxylation reactions [1].
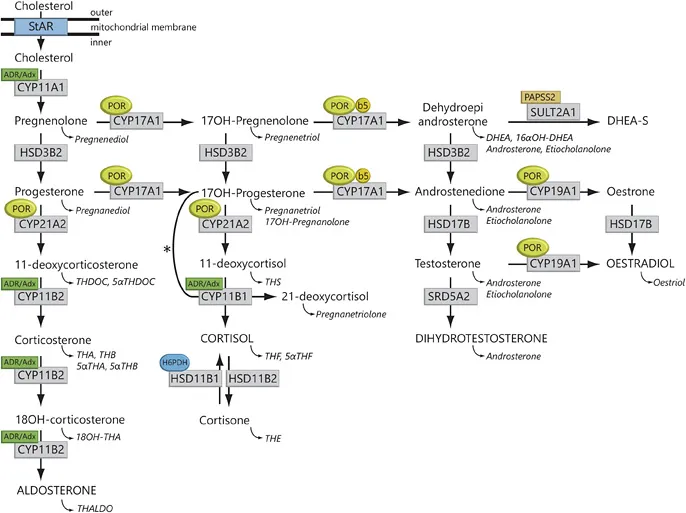
The acute stimulation of steroidogenesis is mediated at the level of cholesterol import into mitochondria, which is facilitated by the steroidogenic acute regulatory (StAR) protein [2]. Thereafter, the first step of steroid hormone biosynthesis is the conversion of cholesterol to pregnenolone catalysed by the mitochondrial P450 side-chain cleavage enzyme (P450scc, CYP11A1; fig. 1).
Fig. 1. Steroidogenesis. After the StAR protein-mediated uptake of cholesterol into mitochondria, aldosterone, Cortisol and androgens are synthesized through the co-ordinated action of a series of steroidogenic enzymes in a zone-specific fashion. The mitochondrial CYP type I enzymes (CYP11A1, CYP11B1, CYP11B2) requiring electron transfer via adrenodoxin reductase (ADR) and adrenodoxin (Adx) are marked (box labelled ‘ADR/Adx’). The microsomal CYP II enzymes (CYP17A1, CYP21A2, CYP19A1) receive electrons from POR (indicated by circled POR). In addition to POR, the 17,20-lyase reaction catalysed by CYP17A1 also requires CYB5 (indicated by circled b5). Urinary steroid hormone metabolites are given in italics below the plasma hormones. The asterisk (*) indicates the11β-hydroxylation of 17OHP to 21-deoxycortisolin 21 OHD. The adrenal conversion of androstenedione to testosterone is catalysed by AKR1C3 (HSD17B5), whereas the testicular conversion is facilitated by HSD17B3. SULT2A1 = Sulfotransferase 2A1; PAPPS2 = 3'-phosphoadenosine5'-phosphosulfate synthase 2; DHEA-S = dehydroepiandrosterone sulphate.
The StAR/CYP11 A1 system is the quantitative regulator of steroidogenesis, whereas the CYP17A1 enzyme represents the qualitative regulator determining the class of produced steroid hormone. In the zona glomerulosa, absence of CYP17A1 leads to mineralocorticoid synthesis. In contrast, isolated 17α-hydroxylase activity of CYP17A1 results in the synthesis of glucocorticoids in the zona fasciculata. Combined activities of CYP17A1 catalyse both the 17α-hydroxylation and 17,20-lyase reaction of 21-carbon (C21) steroids to 19-carbon (C19) precursors of sex steroids in the adrenal zona reticularis and gonads.
The main pathway to sex steroids facilitated by CYP17A1 is the conversion of 17α-hydroxypregnenolone (17Preg) to dehydroepiandrosterone (DHEA; ∆5 pathway), whereas the conversion of 17α-hydroxyprogesterone (17OHP) to androstene-dione (∆4 pathway) is hardly relevant under physiological circumstances. The significant predominance of the ∆5 pathway via DHEA in humans is explained by substrate preference of CYP17A1 for 17Preg. The catalytic efficiency of this conversion is about 100-fold higher for the ∆5-steroid 17Preg than for the ∆4-steroid 17OHP. For the 17,20-lyase reaction, CYP17A1 crucially relies on cytochrome b5 (CYB5), which is expressed in the gonads and in the adrenal zona reticularis at the onset of adrenarche [2]. CYB5 acts as an allosteric factor that fosters the interactions of POR with CYP17A1, enhancing 17,20-lyase activity without influencing 17-hydroxylase activity. Another major physiological role of CYB5 is the reduction of methaemoglobin.
DHEA is further converted by 3β-hydroxysteroid dehydrogenase (HSD) type 2 (HSD3B2) in the gonads and adrenals to androstenedione. In addition, HSD3B2 is also required for the conversion reactions of pregnenolone to progesterone, and 17Preg to l7OHP.
Androstenedione is reduced by 17β-HSD type 3 (HSD17B3) in the testes to testosterone. In androgen target tissues, testosterone is further converted to dihydrotestos-terone (DHT) by steroid 5α-reductase type 2 (SRD5A2) [2].
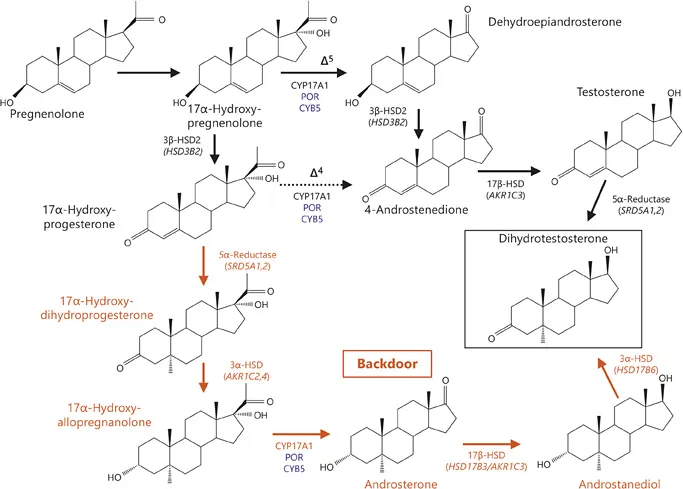
It has recently been suggested that 5α-reduction of testosterone is not the only biosynthetic pathway to produce DHT. In addition, androstanediol can be 3α-oxidated in the target tissues to form DHT via the so-called ‘backdoor pathway’ (fig. 2). In this pathway, androstanediol is hypothesised to derive from sequential 5α- and 3α-reduction of 17OHP to 17α-hydroxyallopregnanolone, followed by its conversion to androsterone by 17,20-lyase activity of CYP 17A1, and then its reduction by HSD17B activity (fig. 2) [3]. The physiological relevance of this pathway remains unclear; however, it is likely to play a significant role in the pathophysiology of distinct conditions such as POR deficiency (PORD) or 21-hydroxylase deficiency (21OHD) [4, 5].
Adrenal 21-hydroxylase (CYP21A2) converts 17OHP to 11 -deoxycortisol and progesterone to 11-deoxycorticosterone (DOC), both precursors of Cortisol and aldosterone biosynthesis. The final steps in the synthesis of glucocorticoids and mineralocorticoids are catalysed by two closely related mitochondrial enzymes, 11β-hydroxylase (CYP11B1) and aldosterone synthase (CYP11B2). Steroid 11β-hydroxylase converts 11-deoxycortisol to cortisol and DOC to corticosterone. CYP11B1 is expressed predominantly in the zona fasciculata, and to a lesser extent in the zona reticularis, but not in the zona glomerulosa. Aldosterone synthase is found only in the zona glomerulosa, where it has 11β-hydroxylase, 18-hydroxylase and 18-methyl oxidase activities; thus, aldosterone synthase is able to catalyse all reactions required for the conversion of DOC to aldosterone [2].
Fig. 2. Pathways to androgen synthesis. Conventional pathways are shown in black, whereas the backdoor pathway is shown in red. Cofactors are shown in blue. The conversion of 17OHP to 17α-hydroxyallopregnanolone is mediated by sequential 5α- and 3α-reduction. In humans, type 1 3α-HSD (encoded by AKR1C4), which is expressed mainly in the liver, but also in the foetal and adult adrenal, is the major reductive 3α-HSD. Additionally, the type 3 3α-HSD (encoded by AKR1C2) also catalyses the reduction of 17α-hydroxydihydroprogesterone to 17α-hydroxyallopregnanolone. The next step on the backdoor route to DHT is the conversion of the 21-carbon steroid 17α-hydroxyallopregnanolone into the 19-carbon androgen androsterone by the 17,20-lyase activity of CYP17A1. Next, androsterone is reduced to androstanediol. Beside the type 3 17β-HSD (encoded by HSD17B3), which is dominantly expressed in the testes, type 2 3α-HSD (also known as type 5 17β-HSD; encoded by AKR1C3) shows a pattern of activity similar to that of the testicular type 3 17β-HSD and additionally reduces androsterone to androstanediol with high catalytic efficiency in humans. AKR1C3 is expressed in many different tissues, such as liver, fat tissue and adrenal cortex, and is responsible for the formation of androgens in women. The 3-hydroxyepimerase (also known as retinol/sterol dehydrogenase; encoded by HSD17B6), with the highest activity levels found in liver and testis, and lower levels in lung, spleen, brain, kidney and ovary, oxidizes androstanediol to DHT in humans [2, 4].
Measurement of Steroids in Body Fluids
The accurate analysis of steroid hormones and their m...