![]()
Vona B, Haaf T (eds): Genetics of Deafness. Monogr Hum Genet. Basel, Karger, 2016, vol 20, pp 110-131
(DOI: 10.1159/000444569)
______________________
Using Zebrafish to Study Human Deafness and Hearing Regeneration
Gaurav K. Varshney1 · Wuhong Pei1 · Shawn M. Burgess
Translational and Functional Genomics Branch, National Human Genome Research Institute, National Institutes of Health, Bethesda, Md., USA
______________________
Abstract
Since the publication of the first draft of the human genome sequence in 2001, there has been an explosion in the number of genes associated with human genetic diseases, including those involved in human deafness. Clinical studies, genome-wide association studies, and exome resequencing have all added to the ever-expanding candidate list of genes with a role in hearing. Because human genetic data is primarily correlative, this explosion of data has increased the need for more efficient approaches to confirm these candidate genes in a model system. In addition, as our understanding of stem cells and genome editing advances, the potential for restoring hearing through regenerative medicine increases. This review highlights the role zebrafish can play as a model for human deafness, and also its potential role in discovering regenerative medicine therapies to restore lost hearing.
© 2016 S. Karger AG, Basel
Hearing loss is one of the most common quality-of-life pathologies affecting the human population. Approximately 2-3 of every 1,000 newborns have a detectable level of hearing loss in one or both ears, and more than 10% of the American population over 18 years of age are suffering with some level of hearing loss, with a clear progression in hearing loss as the person ages (National Institute on Deafness and Other Communication Disorders, http://www.nidcd.nih.gov/health/statistics/Pages/quick.aspx). At age 70 and above, over 50% of the population has measurable hearing loss.
Hearing is mediated by a sensory epithelium containing acoustic mechanosensory receptors called hair cells and a second cell type known as supporting cells located in the cochlea of the inner ear [1]. This epithelium converts sound waves into electrical impulses ultimately interpreted as hearing in the brain. Hearing loss can be caused by many factors such as exposure to loud noise (https://www.nidcd.nih.gov/staticresources/health/hearing/NIDCD-Noise-Induced-Hearing-Loss.pdf), ototoxic chemicals (aminoglycoside antibiotics, cisplatin) [2], infection [3], or head injury [4, 5]. Genetic factors are likely to play an important role in disease pathogenesis, and recent work has attempted to identify genes that make individuals more susceptible to progressive hearing loss [6].
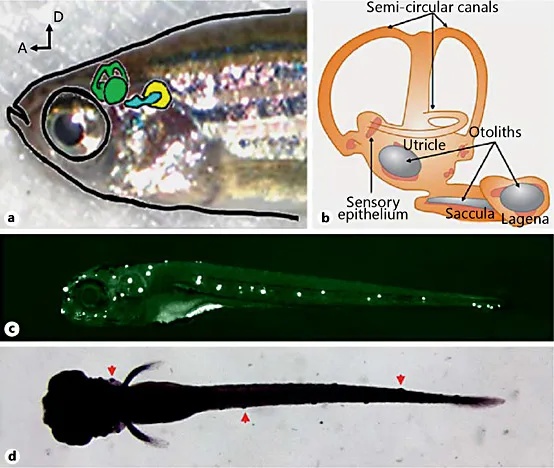
Like most fish, zebrafish have two related sensory organs that utilize the mechanosensory hair cell receptors: (1) the inner ear similar to mammalian ears used to detect sound, gravity, and motion, and (2) the lateral line [7], a fish- and amphibian-specific organ used to detect water flow over the surface of the body (fig. 1). The neuromast consists of clusters of hair cells and supporting cells along the side of the body with each cell type having similar molecular signature to the matching cells in the mammalian inner ear.
Fig. 1. The inner ear and the lateral line in zebrafish. a A bright field image of an adult zebrafish head, with inner ear structure illustrated. The adult fish is oriented in a lateral view, with the nose on the left. The utricle and semicircular canals are highlighted in green, the saccula in blue, and the lagena in yellow. b A cartoon illustration of the structure of the adult zebrafish inner ear. The utricle is involved in vestibular functions, the saccula is primarily hearing, and the lagena is an indeterminate mixture of hearing and vestibular functions. c A fluorescent image of a zebrafish larva, with hair cells stained using the fluorescent dye Yopro-1. The larva is oriented in a lateral view, with head on the left. Each white dot along the body and head is a cluster of hair cells centered in a neuromast. d A bright field image of a zebrafish larva. The larva is oriented in a ventral view, with head on the left. Arrows point to examples of the hair cell-containing neuromasts localized superficially on the skin (visible as small bumps). A = Anterior; D = dorsal.
Intense sound or toxic chemical exposure can damage or kill hair cells which are often more sensitive to injury than the surrounding epithelium [8, 9]. In mammals, these sensory hair cells can only repair themselves if the damage is modest; however, if the damage is significant, apoptosis is triggered in the hair cells. Only non-mammalian vertebrates such as fish or frogs can fully regenerate damaged hearing. In mammals, such as humans, the damage is permanent. Typically, in fish, the supporting cells re-enter mitosis and hair cells differentiate from these activated supporting cells, rapidly and completely restoring full hearing [7]. In recent years, zebrafish has been increasingly used as a model for human disease, including human deafness, because its genome has been fully sequenced [10], and targeted gene inactivation has become commonplace [11]. Zebrafish are also advantageous in terms of visualizing defects in the inner ear as their eggs are fertilized externally and the larvae are optically transparent for the first several days of development, allowing direct observation of the sensory epithelium of the inner ear. In mouse, sensory hair cells are not as directly accessible for either observation or manipulation, and dissection of the inner ear is technically challenging. Because of this general utility, zebrafish is being increasingly used as a model system for human deafness and for studying the factors involved in hearing regeneration. There are many reviews outlining the development of hair cells in zebrafish [12-16]; therefore, in this review, we will focus on recent advances in the use of zebrafish to directly model known human deafness genes and recent advances in our understanding of hearing regeneration in non-mammalian vertebrates.
Zebrafish as a Model for Human Deafness
In nonsyndromic deafness, hearing loss is not accompanied by other symptoms. Approximately 2 of 3 genetic hearing loss conditions are nonsyndromic in nature. According to the Hereditary Hearing loss website (http://hereditaryhearingloss.org), there are more than 100 genes associated with nonsyndromic hearing loss. Of the nonsyndromic deafness cases, 70% are classified as autosomal recessive deafness (DFNB) and 20% as autosomal dominant deafness (DFNA). The remaining cases are caused by X-linked or mitochondrial genetic mutations. In syndromic deafness, the hearing loss is usually associated with other clinical manifestations such as blindness or dysmorphic craniofacial features. The syndromic forms of deafness account for one-third of all hearing loss cases.
In most cases, the genetic evidence for deafness in humans is very good; however, functional validation for most of the candidate genes has not been done. The most common method of validating candidate gene function is to generate a gene knockout in the homologous gene in a model organism; for example, 70% of human genes have orthologs in zebrafish [10]. We have summarized all genes in zebrafish that have an orthologous human gene related to nonsyndromic deafness in table 1. Several large-scale mutagenesis screens have identified multiple genes essential for balance and hearing in zebrafish [17-20]. The screens mainly identified genes based on morphology such as otic placode specification, otolith formation, size and shape of otic vesicle, etc. Approximately 40 such genes were identified for inner ear development [18, 21]. A second approach was based on screening locomotion and behavior of larvae that had normal otic vesicle morphology. This approach identified 17 mutants representing 9 genes where mutations caused the ‘circler’ phenotype. These mutants swam in an erratic fashion when provided with tactile stimulation and did not display an escaping startle response when provided with an acoustic tapping sound. This group of mutants was called the circler mutants [19]. Eight of the circler mutants (sputnik, mariner, orbiter, mercury, gemini, skylab, astronaut, and cosmonaut) were further investigated for vestibular function def...