
eBook - ePub
Arrow-Pushing in Organic Chemistry
An Easy Approach to Understanding Reaction Mechanisms
Daniel E. Levy
This is a test
- English
- ePUB (disponibile sull'app)
- Disponibile su iOS e Android
eBook - ePub
Arrow-Pushing in Organic Chemistry
An Easy Approach to Understanding Reaction Mechanisms
Daniel E. Levy
Dettagli del libro
Anteprima del libro
Indice dei contenuti
Citazioni
Informazioni sul libro
Organic chemistry is required coursework for degrees in life, food, and medical sciences. To help the students discouraged by the belief that this topic cannot be mastered without significant memorization, Arrow Pushing in Organic Chemistry serves as a handy supplement for understanding the subject. • Includes new chapters, an expanded index, and additional problem sets complete with detailed solutions
• Focuses on understanding the mechanics and logic of organic reaction mechanisms
• Introduces ionic and non-ionic reactive species and reaction mechanisms
• Teaches strategies to predict reactive species, sites of reactions, and reaction products
• Provides a solid foundation upon which organic chemistry students can advance with confidence
Domande frequenti
Come faccio ad annullare l'abbonamento?
È semplicissimo: basta accedere alla sezione Account nelle Impostazioni e cliccare su "Annulla abbonamento". Dopo la cancellazione, l'abbonamento rimarrà attivo per il periodo rimanente già pagato. Per maggiori informazioni, clicca qui
È possibile scaricare libri? Se sì, come?
Al momento è possibile scaricare tramite l'app tutti i nostri libri ePub mobile-friendly. Anche la maggior parte dei nostri PDF è scaricabile e stiamo lavorando per rendere disponibile quanto prima il download di tutti gli altri file. Per maggiori informazioni, clicca qui
Che differenza c'è tra i piani?
Entrambi i piani ti danno accesso illimitato alla libreria e a tutte le funzionalità di Perlego. Le uniche differenze sono il prezzo e il periodo di abbonamento: con il piano annuale risparmierai circa il 30% rispetto a 12 rate con quello mensile.
Cos'è Perlego?
Perlego è un servizio di abbonamento a testi accademici, che ti permette di accedere a un'intera libreria online a un prezzo inferiore rispetto a quello che pagheresti per acquistare un singolo libro al mese. Con oltre 1 milione di testi suddivisi in più di 1.000 categorie, troverai sicuramente ciò che fa per te! Per maggiori informazioni, clicca qui.
Perlego supporta la sintesi vocale?
Cerca l'icona Sintesi vocale nel prossimo libro che leggerai per verificare se è possibile riprodurre l'audio. Questo strumento permette di leggere il testo a voce alta, evidenziandolo man mano che la lettura procede. Puoi aumentare o diminuire la velocità della sintesi vocale, oppure sospendere la riproduzione. Per maggiori informazioni, clicca qui.
Arrow-Pushing in Organic Chemistry è disponibile online in formato PDF/ePub?
Sì, puoi accedere a Arrow-Pushing in Organic Chemistry di Daniel E. Levy in formato PDF e/o ePub, così come ad altri libri molto apprezzati nelle sezioni relative a Ciencias físicas e Química orgánica. Scopri oltre 1 milione di libri disponibili nel nostro catalogo.
Informazioni
Chapter 1
Introduction
The study of organic chemistry focuses on the chemistry of elements and materials essential for the existence of life. In addition to carbon, the most common elements present in organic molecules are hydrogen, oxygen, nitrogen, sulfur, and various halogens. Through the study of organic chemistry, our understanding of the forces binding these elements to one another and how these bonds can be manipulated are explored. In general, our ability to manipulate organic molecules is influenced by several factors that include the nature of functional groups near sites of reaction, the nature of reagents utilized in reactions, and the nature of potential leaving groups. In addition, these three factors impart further variables that influence the course of organic reactions. For example, the nature of the reagents used in given reactions can influence the reaction mechanisms and ultimately the reaction products. By recognizing the interplay between these factors and by applying principles of arrow‐pushing, which in reality represents bookkeeping of electrons, reasonable predictions of organic mechanisms and products can be realized without the burden of committing to memory the wealth of organic reactions studied in introductory courses. In this chapter, the concept of arrow‐pushing is defined in context with various reaction types, functional groups, mechanism types, reagents/nucleophiles, and leaving groups.
1.1 DEFINITION OF ARROW‐PUSHING
Organic chemistry is generally presented through a treatment of how organic chemicals are converted from starting materials to products. For example, the Wittig reaction (Scheme 1.1) is used for the conversion of aldehydes and ketones to olefins, and the Diels–Alder reaction (Scheme 1.2) is used for the formation of six‐membered ring systems and treatment of alkyl halides with reagents such as tributyltin hydride (Scheme 1.3), resulting in removal of the associated halides. However, by presenting these reactions as illustrated in Schemes 1.1, 1.2, and 1.3, no explanation is provided as to how the starting materials end up as their respective products.
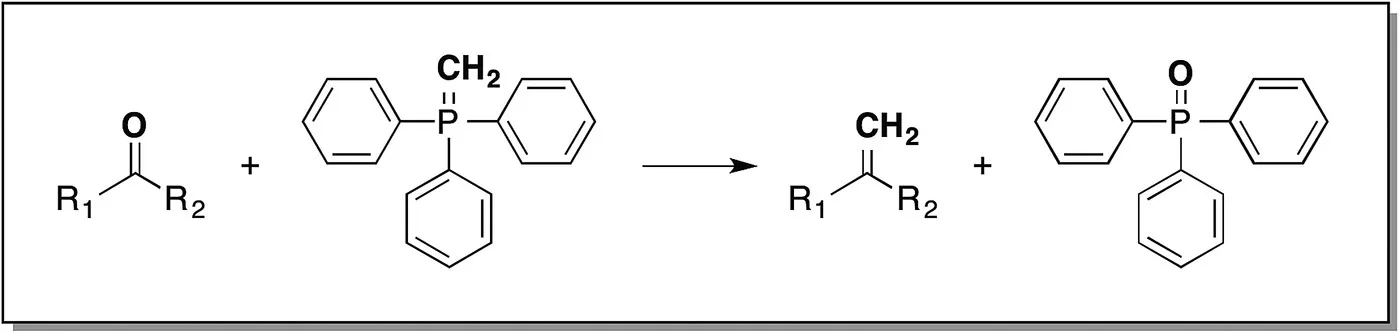
Scheme 1.1 Example of the Wittig reaction.
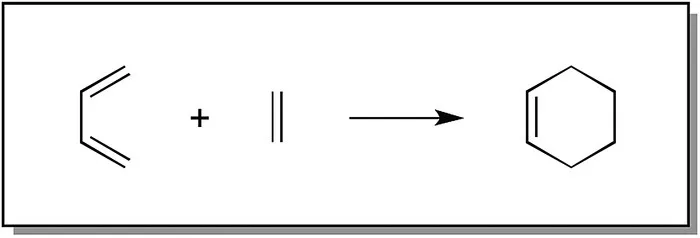
Scheme 1.2 Example of the Diels–Alder reaction.
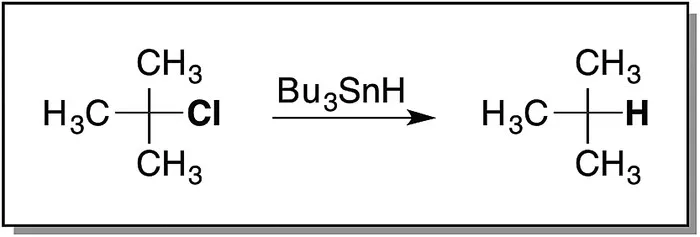
Scheme 1.3 Example of a tin hydride dehalogenation.
By definition, the outcome of any chemical reaction is the result of a process resulting in the breaking and formation of chemical bonds. Referring to material covered in most general chemistry courses, bonds between atoms are defined by sets of two electrons. Specifically, a single bond between two atoms is made of two electrons, a double bond between ato...