Introduction to Reticular Chemistry
Metal-Organic Frameworks and Covalent Organic Frameworks
Omar M. Yaghi, Markus J. Kalmutzki, Christian S. Diercks
- English
- ePUB (disponibile sull'app)
- Disponibile su iOS e Android
Introduction to Reticular Chemistry
Metal-Organic Frameworks and Covalent Organic Frameworks
Omar M. Yaghi, Markus J. Kalmutzki, Christian S. Diercks
Informazioni sul libro
A concise introduction to the chemistry and design principles behind important metal-organic frameworks and related porous materials Reticular chemistry has been applied to synthesize new classes of porous materials that are successfully used for myraid applications in areas such as gas separation, catalysis, energy, and electronics. Introduction to Reticular Chemistry gives an unique overview of the principles of the chemistry behind metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and zeolitic imidazolate frameworks (ZIFs). Written by one of the pioneers in the field, this book covers all important aspects of reticular chemistry, including design and synthesis, properties and characterization, as well as current and future applications Designed to be an accessible resource, the book is written in an easy-to-understand style. It includes an extensive bibliography, and offers figures and videos of crystal structures that are available as an electronic supplement. Introduction to Reticular Chemistry: -Describes the underlying principles and design elements for the synthesis of important metal-organic frameworks (MOFs) and related materials
-Discusses both real-life and future applications in various fields, such as clean energy and water adsorption
-Offers all graphic material on a companion website
-Provides first-hand knowledge by Omar Yaghi, one of the pioneers in the field, and his team. Aimed at graduate students in chemistry, structural chemists, inorganic chemists, organic chemists, catalytic chemists, and others, Introduction to Reticular Chemistry is a groundbreaking book that explores the chemistry principles and applications of MOFs, COFs, and ZIFs.
Domande frequenti
Informazioni
Part I
Metal-Organic Frameworks
1
Emergence of Metal‐Organic Frameworks
1.1 Introduction
1.2 Early Examples of Coordination Solids
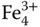
1.3 Werner Complexes