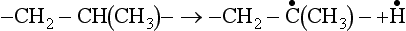INTRODUCTION
People cannot live without oxygen and water. But these are deadly enemies of polymers, both in processing of plastics formulations and in service. Water is a problem mainly for condensation polymers which degrade by hydrolysis. In this paper the focus is on oxidative degradation.
Oxygen degrades polymers to lower molecular weight (MW) by reacting with polymer free radicals to form peroxy free radicals (ROO•) and hydroperoxides (ROOH). Free radicals have an unshared electron and react in any way they can to restore the atom or molecule to a balanced structure. Often that leads to chain scission. As MW goes down most polymer properties suffer. As little as 5-10% reduction in MW may cause failure. Avoiding contact with oxygen and using an antioxidant (AO) as a free radical scavenger are means of preventing degradation.
The high temperature required to process plastics is the major cause of degradation in injection molding, extrusion, blow molding, etc. High temperature is needed to fuse polymers and to reduce melt viscosity to a level that the machines can handle. Mechanical shear of the melt and the presence of oxygen, even in small amounts, are major factors in degradation due to processing. The chain carbon atoms attached to a branch, such as methyl group (CH3), tend to split off a hydrogen atom, creating a free radical at a tertiary carbon atom.
Very little oxygen is needed to react with free radicals during processing. Polymer suppliers usually have very little AO in the resin as sold to processors. Unless additional AO is added, polymer is likely to degrade in process. Polyolefins, which have only carbon-carbon chain bonds (PE, PP, EP and other copolymers) are particularly susceptible to oxidative degradation, in service as well as in processing. Even if additional AO is added, severe processing conditions (high temperature, high shear, long residence time in the barrel), use of regrind, etc. may deplete most of the AO, leaving too little to withstand conditions in service.
Even in moderate service conditions, such as a PE eyewash squeeze bottle on a laboratory wall, oxidative degradation can lead to failure in long term applications. Such a PE bottle, which had been on a laboratory wall for 15-20 years, cracked when tested in a safety inspection. Here, too, additional AO is needed to survive many years of service.
A complicating factor in processing is formulations containing peroxides to crosslink the polymer. Peroxide causes crosslinking by decomposing to free radicals (ROOR → 2RO). The high content of peroxy free radicals formed abruptly reacts with the polymer to cause crosslinking. These free radicals may react with the AO, leaving the system without enough AO for the polymer to survive processing and service. The AO system must be chosen accordingly.
Commonly used methods of analysis to determine if failure is due to oxidative degradation are differential scanning calorimetry (DSC) for oxidative induction time (OIT), (ASTM D3895) or oxidative induction temperature (ASTM D3350). Infrared spectroscopy (IR) may detect bound oxygen as carbonyl (C=O), which forms increasingly as AO becomes exhausted. A third method is change in MW measured as an increase in melt flow rate (MFR), (ASTM D1238). This is a very practical method because it relates directly to MW, i.e., a small reduction in MW gives a large increase in MFR. The applicable relationship is n=KM3.4. Gel permeation chromatography (GPC) is also useful for monitoring MW changes in processing or service.
The DSC methods require about an hour or less, after establishing test conditions, and are most useful for comparing materials, e.g., before and after processing, or after service. They are a practical method of determining the relative amount of AO remaining. When a sample’s AO content is zero, oxidation exotherm starts very soon after oxygen is admitted into the DSC cell. Additional information on the DSC methods is given in the next section. IR is useful mainly to detect bound oxygen, which occurs when most or all of the AO has been depleted.
Examples are given below of failure due to oxidative degradation for (1) HDPE power cable jacket; (2) PE low voltage cable in a power plant control room; (3) PP rotors in a hot water system; (4) EPDM hot water check valve; and (5) EVA (ethylene vinylacetate) hot melt adhesive degraded in a heated reservoir.
A recent case of PP failure in hot water heaters, most likely due to oxidative degradation, was reported in Consumer Reports, July 1999, p. 8. PP that replaced copper dip tubes brings cold water to the bottom of the heater. The PP has been disintegrating into small pieces and clogging pipes and other water delivery systems, and preventing normal operation of the hot water heater. Class action law suits have been filed in some states. Sixteen million heaters were made between 1993 and 1996 with PP dip tubes that may be defective.