
Methods and Applications of Cycloaddition Reactions in Organic Syntheses
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Methods and Applications of Cycloaddition Reactions in Organic Syntheses
About this book
Advanced tools for developing new functional materials and applications in chemical research, pharmaceuticals, and materials science
Cycloadditions are among the most useful tools for organic chemists, enabling them to build carbocyclic and heterocyclic structures. These structures can then be used to develop a broad range of functional materials, including pharmaceuticals, agrochemicals, dyes, and optics. With contributions from an international team of leading experts and pioneers in cycloaddition chemistry, this book brings together and reviews recent advances, trends, and emerging research in the field.
Methods and Applications of Cycloaddition Reactions in Organic Syntheses focuses on two component cycloadditions, with chapters covering such topics as:
- N1 unit transfer reaction to C–C double bonds
- [3+2] Cycloaddition of ?, ?-unsaturated metal-carbene complexes
- Formal [3+3] cycloaddition approach to natural product synthesis
- Development of new methods for the construction of heterocycles based on cycloaddition reaction of 1,3-dipoles
- Cycloreversion approach for preparation of large ?-conjugated compounds
- Transition metal-catalyzed or mediated [5+1] cycloadditions
Readers will learn methods for seamlessly executing important reactions such as Diels-Alder and stereoselective dipolar reactions in order to fabricate heterocyclic compounds, natural products, and functional molecules. The book not only features cutting-edge topics, but also important background information, such as the contributors' process for developing new methodologies, to help novices become fully adept in the field. References at the end of each chapter lead to original research papers and reviews for facilitating further investigation of individual topics.
Covering the state of the science and technology, Methods and Applications of Cycloaddition Reactions in Organic Syntheses enables synthetic organic chemists to advance their research and develop new functional materials and applications in chemical research, pharmaceuticals, and materials science.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1.1 Introduction
1.2 Cyclopropanation Reaction Via Michael-Induced Ring Closure Reaction
1.2.1 Introduction
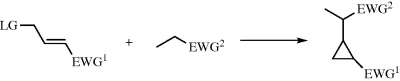
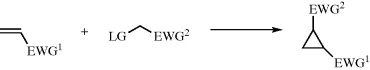
Table of contents
- Cover
- Title Page
- Copyright
- Preface
- Contributors
- Part I: [2+1] Cycloaddition
- Part II: [2+2] Cycloaddition
- Part III: [2+2] and [4+2]/[2+2] Cycloaddition
- Part IV: [3+2] Cycloaddition
- Part V: [3+2], [3+3], and [4+2] Cycloaddition
- Part VI: [3+2] and [5+1] Cycloaddition
- Part VII: [3+3] Cycloaddition
- Part VIII: [4+2] Cycloaddition
- Part IX: [4+2]/[3+2] Cycloaddition
- Part X: [5+1] Cycloaddition
- Part XI: [4+3] Cycloaddition
- Part XII: [5+2] Cycloaddition
- Index