
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
Toxicology is the study of the adverse effects of chemical, physical, or biological agents on people, animals, and the environment. Toxicologists are trained to investigate, interpret, and communicate the nature of those effects.
Over the last ten years the subject of toxicology has changed dramatically, moving from a discipline which was once firmly wedded to traditional methods to one which is keen to embrace the innovative techniques emerging from the developing fields of cell culture and molecular biology. There is an acute need for this to be reflected in a paradigm shift which takes advantage of the opportunities offered by modern developments in the life sciences, including new in vitro and in silico approaches, alternative whole organism (non-mammalian) models and the exploitation of 'omics methods, high throughput screening (HTS) techniques and molecular imaging technologies.
This concise, accessible introduction to the field includes the very latest concepts and methodologies. It provides MSc, PhD and final year undergraduate students in pharmacy, biomedical and life sciences, as well as individuals starting out in the cosmetics, consumer products, pharmaceutical and testing industries, with everything they need to know to get to grips with the fast moving field of toxicology and the current approaches used in the risk assessment of drugs and chemicals.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Chapter 1
Background to Molecular and Cellular Toxicology
1.1 What do we mean by molecular and cellular toxicology?
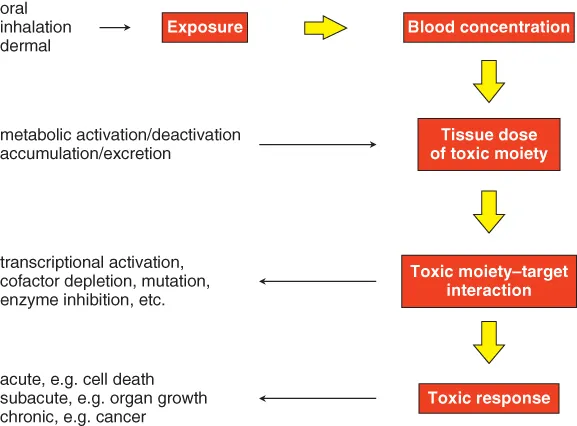
1.2 Tissues and their maintenance
- The endoderm gives rise to the epithelia of the gut and its associated organs (lung, liver and pancreas).
- The ectoderm gives rise to the outer surface epithelia (epidermis, buccal epithelium and outer cervical epithelium) and neuroectodermal tissues.
- The mesoderm gives rise to the embryonic mesenchyme and thence to the connective tissue and supporting tissues including bone, cartilage, muscle, vascular tissue and haematopoietic system.
1.2.1 Stem cells
- A totipotent stem cell can generate an entire new organism. The definitive totipotent stem cell is the fertilised egg; following implantation, the totipotent fertilised egg becomes committed to form an embryonic pluripotent stem cell.
- A pluripotent stem cell can give rise to any other type of cell but not to an entire new organism. Pluripotent cells give rise to committed progenitor cells which can only mature into one type of cell (i.e. each one is unipotent) and this maturation process involves differentiation, which is controlled by growth factors and the surrounding environment.
- Multi-potent stem cells can produce a limited number of cell types and are committed to become part of a particular organ. They give rise to lineages of progenitor cells.
- Progenitor cells are committed to a particular lineage (e.g. the haematopoietic system) and give rise to terminally differentiated cells, which do not divide further.
1.3 Tissue damage
- Primary (direct) injury involves an interaction between the chemicals and the components of the cell. Toxic cell injury requires high concentrations of toxic compounds and, in some cases, metabolic activation. It may involve membrane damage (e.g. lipid peroxidation induced by carbon tetrachloride in the liver).
- Secondary (indirect) injury involves changes in the cellular environment (e.g. oxygen tens...
Table of contents
- Cover
- Title Page
- Copyright
- Dedication
- Foreword
- Preface
- References
- Acknowledgements
- Abbreviations
- About the Companion Website
- Chapter 1: Background to Molecular and Cellular Toxicology
- Chapter 2: Individual Susceptibility to Toxic Chemicals
- Chapter 3: ‘Omics Techniques
- Chapter 4: In Vitro Methods for Predicting In Vivo Toxicity
- Chapter 5: In Vitro Methods for Absorption, Distribution, Metabolism and Excretion
- Chapter 6: In Silico Methods and Structure–Activity Relationships
- Chapter 7: Transgenic Animal Models for ADME and Systemic Toxicity
- Chapter 8: Genotoxicity and its Measurement
- Chapter 9: Oncogenes and the Identification of Human Carcinogens
- Chapter 10: Emerging Techniques
- Index
- End User License Agreement