
Biopharmaceutics Modeling and Simulations
Theory, Practice, Methods, and Applications
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Biopharmaceutics Modeling and Simulations
Theory, Practice, Methods, and Applications
About this book
A comprehensive introduction to using modeling and simulation programs in drug discovery and development
Biopharmaceutical modeling has become integral to the design and development of new drugs. Influencing key aspects of the development process, including drug substance design, formulation design, and toxicological exposure assessment, biopharmaceutical modeling is now seen as the linchpin to a drug's future success. And while there are a number of commercially available software programs for drug modeling, there has not been a single resource guiding pharmaceutical professionals to the actual tools and practices needed to design and test safe drugs.
A guide to the basics of modeling and simulation programs, Biopharmaceutics Modeling and Simulations offers pharmaceutical scientists the keys to understanding how they work and are applied in creating drugs with desired medicinal properties. Beginning with a focus on the oral absorption of drugs, the book discusses:
- The central dogma of oral drug absorption (the interplay of dissolution, solubility, and permeability of a drug), which forms the basis of the biopharmaceutical classification system (BCS)
- The concept of drug concentration
- How to simulate key drug absorption processes
- The physiological and drug property data used for biopharmaceutical modeling
- Reliable practices for reporting results
With over 200 figures and illustrations and a peerless examination of all the key aspects of drug research—including running and interpreting models, validation, and compound and formulation selection—this reference seamlessly brings together the proven practical approaches essential to developing the safe and effective medicines of tomorrow.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1.1 An Illustrative Description of Oral Drug Absorption: The Whole Story










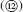
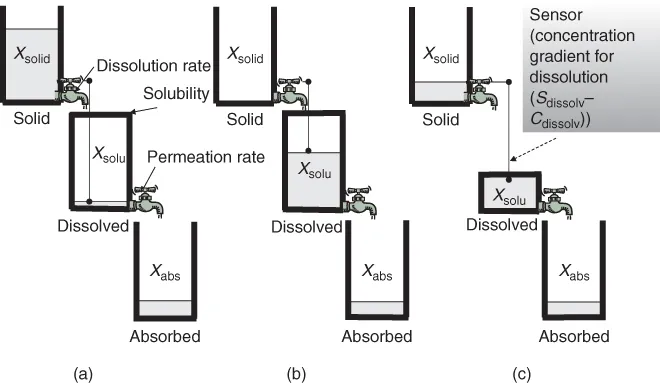
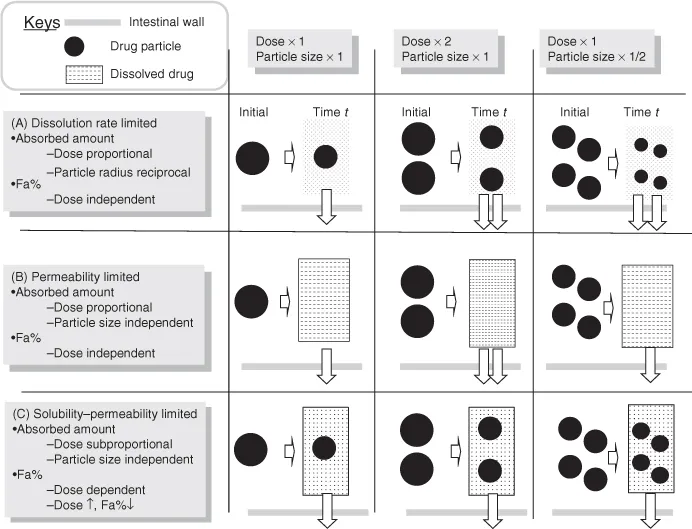
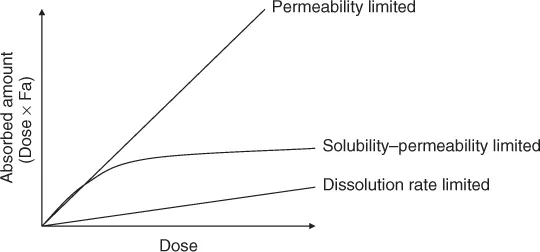
1.2 Three Regimes of Oral Drug Absorption
- Dissolution rate-limited absorption (DRL) (Fig. 1.2a)
- Permeability-limited absorption (PL) (Fig. 1.2b)
- Solubility-permeability-limited absorption (SL) (Fig. 1.2c)
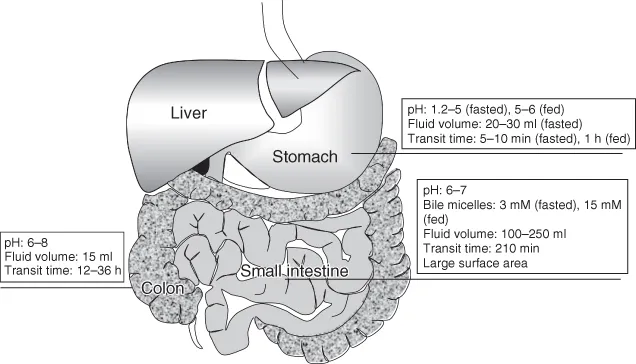
1.3 Physiology of the Stomach, Small Intestine, and Colon
1.4 Drug and API Form
Table of contents
- Cover
- Title
- Copyright
- Dedication
- Preface
- List of Abbreviations
- Chapter 1: Introduction
- Chapter 2: Theoretical Framework I: Solubility
- Chapter 3: Theoretical Framework II: Dissolution
- Chapter 4: Theoretical Framework III: Biological Membrane Permeation
- Chapter 5: Theoretical Framework IV: Gastrointestinal Transit Models and Integration
- Chapter 6: Physiology of Gastrointestinal Tract and other Administration Sites in Humans and Animals
- Chapter 7: Drug Parameters
- Chapter 8: Validation of Mechanistic Models
- Chapter 9: Bioequivalence and Biopharmaceutical Classification System
- Chapter 10: Dose and Particle Size Dependency
- Chapter 11: Enabling Formulations
- Chapter 12: Food Effect
- Chapter 13: Biopharmaceutical Modeling for Miscellaneous Cases
- Chapter 14: Intestinal Transporters
- Chapter 15: Strategy in Drug Discovery and Development
- Chapter 16: Epistemology of Biopharmaceutical Modeling and Good Simulation Practice
- Appendix A: General Terminology
- Appendix B: Fluid Dynamics
- Index