
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Practical High-Performance Liquid Chromatography
About this book
Three decades on from publication of the 1st German edition of Veronika Meyer's book on HPLC, this classic text remains one of the few titles available on general HPLC aimed at practitioners.
New sections on the following topics have been included in this fifth edition:
- Comparison of HPLC with capillary electrophoresis
- How to obtain peak capacity
- van Deemter curves and other coherences
- Hydrophilic interaction chromatography
- Method transfer
- Comprehensive two-dimensional HPLC
- Fast separations at 1000 bar
- HPLC with superheated water
In addition, two chapters on the instrument test and troubleshooting in the appendix have been updated and expanded by Bruno E. Lendi, and many details have been improved and numerous references added.
A completely new chapter is presented on quality assurance covering:
- Is it worth the effort?
- Verification with a second method
- Method validation
- Standard operating procedures
- Measurement uncertainty
- Qualifications, instrument test, and system suitability test
- The quest for quality
Reviews of earlier editions
"That this text is written by an expert in both the practice and teaching of HPLC is evident from the first paragraph....not only an enjoyable, fascinating and easy read, but a truly excellent text that has and will serve many teachers, students and practitioners very well." —The Analyst
"…provides essential information on HPLC for LC practitioners in academia, industry, government, and research laboratories…a valuable introduction." - American Journal of Therapeutics
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Introduction
1.1 HPLC: A POWERFUL SEPARATION METHOD
1.2 A FIRST HPLC EXPERIMENT
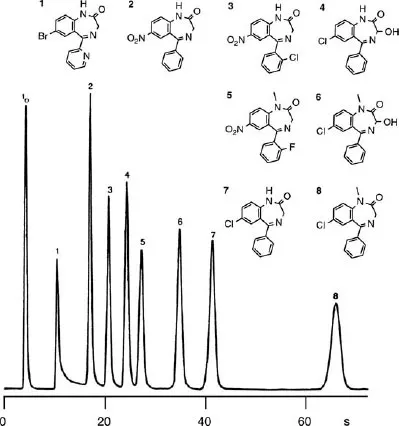
1.3 LIQUID CHROMATOGRAPHIC SEPARATION MODES
Adsorption Chromatography
Table of contents
- Cover
- Table of Contents
- Title
- Copyright
- Dedication
- Preface to the Fifth Edition
- Important and Useful Equations for HPLC
- 1 Introduction
- 2 Theoretical Principles
- 3 Pumps
- 4 Preparation of Equipment up to Sample Injection
- 5 Solvent Properties
- 6 Detectors
- 7 Columns and Stationary Phases
- 8 HPLC Column Tests
- 9 Adsorption Chromatography: Normal-Phase Chromatography
- 10 Reversed-Phase Chromatography
- 11 Chromatography with Chemically Bonded Phases
- 12 Ion-Exchange Chromatography
- 13 Ion-Pair Chromatography
- 14 Ion Chromatography
- 15 Size-Exclusion Chromatography
- 16 Affinity Chromatography
- 17 Choice of Method
- 18 Solving the Elution Problem
- 19 Analytical HPLC
- 20 Quality Assurance
- 21 Preparative HPLC
- 22 Separation of Enantiomers
- 23 Special Possibilities
- 24 Appendix 1: Applied HPLC Theory
- 25 Appendix 2: How to Perform the Instrument Test
- 26 Appendix 3: Troubleshooting
- 27 Appendix 4: Column Packing
- Index of Separations
- Subject Index
- End User License Agreement
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app