![]()
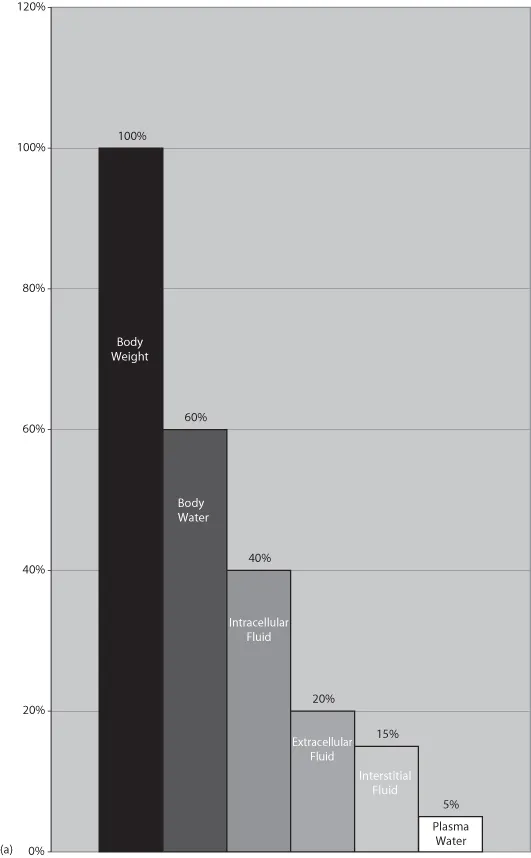
Body water is typically described by referring to how much of an animal’s body weight it represents. It is most often defined as a percentage and thus can be calculated for any patient. In the average healthy animal, total body water is equivalent to 60% of total body weight. An animal’s body condition must be considered before determining the expected volume of body water. Because fat contains little or no water, an obese animal will have less than 60% of its body weight devoted to water. Animals that are excessively thin have a higher percentage of weight, roughly 70% as body water. Neonates also differ from the average healthy adult because water comprises roughly 80% of their body weight (Michell et al., 1989).
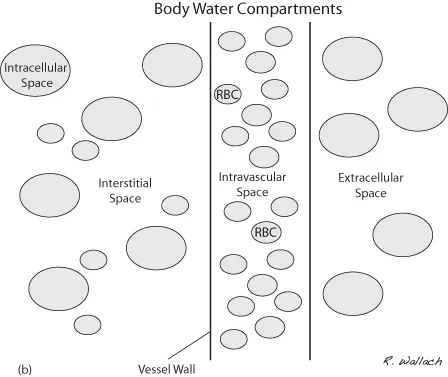
Body water is housed in several different compartments. Of the 60% of body weight that is fluid, 40% is maintained in the intracellular fluid space (ICF) and 20% in the extracellular fluid space (ECF) (Wellman et al., 2006). Although the extracellular space houses a smaller volume of body water than the intracellular space, it is an extremely important consideration during fluid therapy.
In illness, fluid loss occurs through different routes. Initial losses may cause a disturbance in one of the body’s compartments, but these losses eventually are distributed and shared across all compartments (Fig. 1.1A, B). Water is borrowed from one space to replenish another. The compartments that are smallest by comparison experience a more significant impact from their loss than the larger compartments. Because the extracellular space is small to begin with, losses from this space must be addressed, and it is the first compartment that is replenished by intravenous fluid therapy.
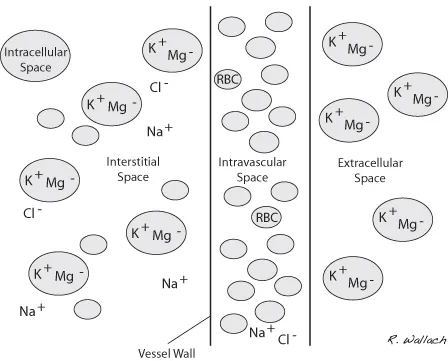
Much of the extracellular fluid is found in the tissues, bathing the cells, and is referred to as interstitial fluid (ISF). This ISF represents approximately three-quarters of the total ECF, translating into about 15% of the patient’s body weight. The remainder of the ECF is found in the vascular space and represents 5% of total body weight. Body water housed within the vasculature is referred to as plasma, the noncellular portion of the blood. At first glance it may seem that these numbers do not play a vital role in the day-to-day routine of the veterinary technician. However, it is important to recognize that without the ability to estimate the fluid volume in the extracellular compartment, we cannot plan appropriate fluid therapy to support or replenish our compromised patients.
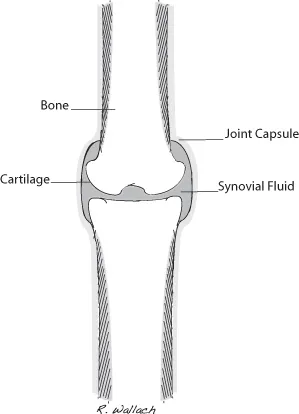
The synovial joints, the aqueous chambers of the eyes, and the cerebrospinal space contain a small portion of ECF (∼1% body weight) and are known as the transcellular fluid compartments (Wellman 2006) (Fig. 1.2).
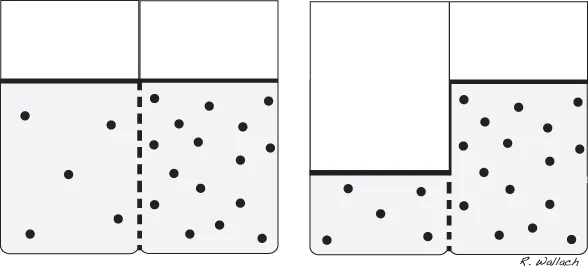
In the healthy animal the movement of fluid throughout these compartments is well controlled. There is a natural tendency for water to move in a manner that will balance the solute concentrations between compartments. Solutes are the dissolved substances that reside within body fluids. When the solute concentration of a compartment is altered, water moves into the compartment with the higher solute concentration to restore balance.
Body water compartments are separated by a semipermeable membrane, which means that water and certain solutes may pass through the membrane and others may not. The process of osmosis occurs when water passes through a semipermeable membrane and is drawn toward a space that has a higher solute concentration (Wellman 2006). Movement of water into an area of higher solute concentration dilutes the solute and reinstates equilibrium.
For the various body water compartments to carry out their physiologic responsibilities, it is sometimes necessary to maintain water within a compartment and prevent its movement into higher solute areas. Various solutes within the compartments are responsible for this regulation. Due to their inability to pass freely through semipermeable membranes, these solutes exert a force on the interior of their respective compartment. This force, referred to as osmotic pressure, is the amount of force required to keep water within a compartment (Guyton and Hall 2000d). A compartment with higher solute concentration has greater osmotic pressure. This compartment exerts the stronger pull (between two compartments) and draws water toward it, across the semipermeable membrane (Fig. 1.3).
Electrolytes and Their Fluid Compartments
The extracellular and intracellular fluid compartments contain different concentrations of important solutes called ions, which are electrically charged particles found throughout the body water compartments. The term electrolyte refers to the combination of ions to form a substance that will break down in water.
Electrolytes have an important role in maintaining acid-base status within the body. A second but no less important role of electrolytes is to provide osmotic pressure and regulate the movement of body water between compartments.
The extracellular and intracellular spaces contain high concentrations of sodium (Na+) and chloride (Cl−) and potassium (K+) and magnesium (Mg++), respectively (Guyton and Hall 2000c) (Fig. 1.4).
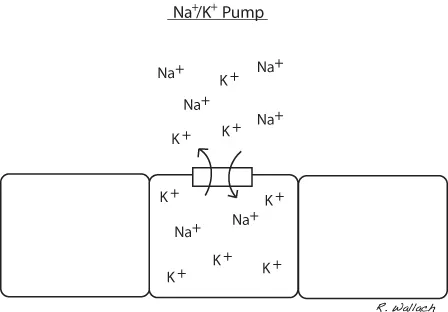
Sodium has a positive electrical charge associated with it and referred to as a cation. It is the most plentiful cation in the ECF. Sodium plays an important role in maintaining the volume of water in the extracellular compartment. It is exchanged between the intracellular and extracellular compartments with ease. Each cell membrane houses a pump that exchanges sodium for potassium. When sodium concentration reaches an unsuitable level within the cell, the sodium potassium pump removes a sodium ion (positive charge) in exchange for a potassium ion (positive charge) (Wellman et al., 2006) (Fig. 1.5). This mechanism maintains a higher concentration of sodium outside the cell, making it an important regulator of osmosis (water will diffuse into areas of higher sodium concentration).
Chloride has a negative charge associated with it and is referred to as an anion. It is the most abundant anion in the ECF compartment. It too can move in and out of cells. The ECF also contains a substantial amount of bicarbonate and a small amount of potassium. Despite their smaller numbers, these ions have important physiologic roles and impart severe consequences when their concentrations are altered.
The ICF compartment is characterized by high concentrations of potassium (cation) and magnesium (anion). Potassium is vital to normal cellular metabolism due to its important influence has on the movement of water into and out of the cell. Disease(s) causing damage to, or death of, cells results in cellular potassium leaking into the extracellular space. This is of particular interest in the clinical setting when we consider how often we see abnormalities in serum potassium levels. Hyperkalemia, the condition in which the level of potassium within the blood is elevated, can lead to severe arrhythmias (Stepien 1999). In particular, bradyarrhythmias and cardiac arrest are significant concerns with hyperkalemic patients. Magnesium, phosphate, and protein molecules also impart a regulatory influence on the movement of water between compartments.
The concentration of electrolytes within a solution can be expressed in several different ways. One of the formats encountered frequently in fluid therapy is millimoles per liter (mmol/L). This measurement denotes the molecular weight of the electrolyte in a volume of solvent (Wellman 2006).
Water Gain and Water Loss
The volume of water that normally exists in the body is fairly consistent. Animals have two primary means of obtaining water: drinking and eating. A normal healthy animal regulates its drinking based on its body’s requirements. For example, the body of an animal that has been exposed to an extremely hot environment is likely to lose a larger volume of body water than normal due to its panting (increased loss of water via respiratory tract) than an animal resting in a cool indoor environment. The loss of water from the ECF compartment lowers the concentration of water and increases solute concentrations in that compartment. This decrease in water concentration in the ECF compartment sets in motion a chain of activities, ultimately resulting in an increased water demand (increased thirst).
In the literature, a wide range of values is published for the volume of water normally consumed by a healthy animal in one day. In canines, water consumption varies between 50 and 100 mL/kg per day, whereas felines are expected to consume somewhere between 40 and 70 mL/kg per day (Wellman 2006). The volume of water obtained through an animal’s diet can vary depending on the type of food consumed. Canned food has a higher water content than dry kibble, and as a result, animals on a canned diet consume less water than those on a dry diet. Semi-moist kibble is also available for canines and felines. This type of diet falls in the middle of the moisture range, containing more water than kibble but less than canned food.
Dietary proteins, carbohydrates, and fats are broken down into smaller blocks as they are digested. Carbohydrates are reduced into simple sugars, fats into fatty acids and glycerol, and protein into amino acids. One of the processes involved in digestion is oxidation, defined as the combination of a substance with oxygen. Water is one of the products of this process and as such is made available to the body through the digestion of food (Guyton and Hall 2000a)...