
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Water Pollution Control
About this book
This book begins with a discussion of the basics of the hydrological cycle and a description of the natural aquatic environment including the normal composition of surface waters. Further chapters detail the sources of water pollution and the affects of water pollution including biological treatment of sewerage, sludge treatment and disposal, before addressing industrial wastewater treatment and water quality assessment.
Discover our e-book series on Environmental Monitoring and Protection, published in partnership with The Open University!
Find out more about the series editors, the titles in the series and their focus on water, noise, air and waste, and The Open University courses in Environmental Management.
Visit www.wiley.com/go/ouebookseries
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Section 1: Water basics
1.1 Introduction
- for cooling – it helps keep our bodies at around 37 °C
- as a waste disposal medium
- as a conductor for nerve impulses
- as a component in the digestion of food
- as a solvent in which vital chemical reactions take place.
1.2 The hydrological cycle

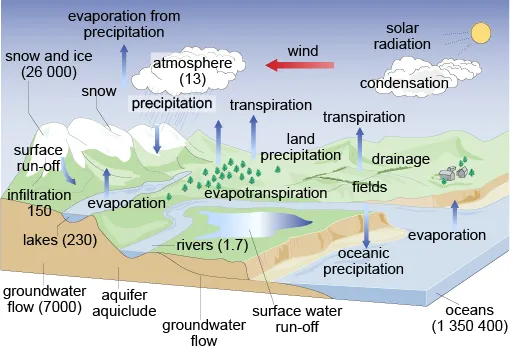
1.3 The natural aquatic environment
1.3.1 Water, the medium of life
Flowing and standing water

Table of contents
- Section 1: Water basics
- Section 2: Pollution of the aquatic environment
- Section 3: The effects of pollutants on the aquatic environment
- Section 4: Sewage treatment
- Section 5: Sludge treatment and disposal
- Section 6: Water quality tests
- Section 7: Industrial wastewater treatment
- Section 8: River quality modelling
- Glossary
- References
- Acknowledgements
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app