
eBook - ePub
Environmental Chemistry and Toxicology of Mercury
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Environmental Chemistry and Toxicology of Mercury
About this book
This book provides the fundamentals, recent developments, and future research needs for critical mercury transformation and transport processes, as well as the experimental methods that have been employed in recent studies. The coverage discusses the environmental behavior and toxicological effects of mercury on organisms, including humans, and provides case studies at the end of each chapter. Bringing together information normally spread across several books, this text is unique in covering the entire mercury cycle and providing a baseline for what is known and what uncertainties remain in respect to mercury cycling.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Environmental Chemistry and Toxicology of Mercury by Guangliang Liu, Yong Cai, Nelson O'Driscoll, Guangliang Liu,Yong Cai,Nelson O'Driscoll in PDF and/or ePUB format, as well as other popular books in Medicine & Toxicology. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Overview of Mercury in the Environment
1.1 Introduction
Mercury (Hg) is a naturally occurring element that is present throughout the environment. Mercury is recognized as a global contaminant because it can undergo long-range transport in the atmosphere, be persistent in the environment, be accumulated in the food web, and pose severe adverse effects on the human and ecosystem health (Nriagu, 1979; Fitzgerald et al., 2007b). The environmental contamination of land, air, water, and wildlife in various ecosystems with mercury around the world due to the natural release and extensive anthropogenic use of Hg has been a global concern for decades (Lindberg and Turner, 1977; Ebinghaus et al., 1999; Fitzgerald et al., 2005; Mason et al., 2009). This being the first chapter of the book, it will briefly discuss the health risks associated with mercury exposure and the natural and anthropogenic sources of mercury emissions, and then provide a very brief overview of the biogeochemical cycling of mercury.
In the environment and in biological systems, mercury can exist in three oxidation states, namely, Hg(0) (metallic), Hg(II) (mercuric), and Hg(I) (mercurous), with the monovalent form being rare owing to its instability (Ullrich et al., 2001; Fitzgerald et al., 2007a,b). In general, the dominant form of mercury in water, soil, and sediment is the inorganic Hg(II) form while methylmercury (MeHg) is dominant in biota, and in the atmosphere Hg(0) is the primary species (USEPA, 1997; Ullrich et al., 2001).
1.2 Toxicity and Health Risks of Mercury Exposure
All forms of mercury are toxic, but particularly problematic are the organic forms such as MeHg, which is a neurotoxin (Committee on the Toxicological Effects of Methylmercury, 2000; Clarkson and Magos, 2006). Acute mercury exposure can produce permanent damage to the nervous system, resulting in a variety of symptoms such as paresthesia, ataxia, sensory disturbances, tremors, blurred vision, slurred speech, hearing difficulties, blindness, deafness, and death (USEPA, 1997; Committee on the Toxicological Effects of Methylmercury, 2000; Clarkson and Magos, 2006). In addition to neurotoxicity, mercury, in inorganic and/or organic forms, can affect other systems and sequentially cause adverse effects including renal toxicity, myocardial infarction, immune malfunction, and irregular blood pressure (USEPA, 1997; Committee on the Toxicological Effects of Methylmercury, 2000).
Human exposure to Hg can pose a variety of health risks, with the severity depending largely on the magnitude of the dose. Historically, there were two notorious poisoning episodes associated with the extremely high MeHg exposures, that is, in Minamata where individuals were poisoned by MeHg through consumption of contaminated fish and in Iraq where the consumption of MeHg-treated (as a fungicide) grain led to poisoning (Committee on the Toxicological Effects of Methylmercury, 2000). Nowadays, acute poisoning incidents from high Hg exposure are rare and the health risks mercury poses to human population are mainly from chronic MeHg exposure through consumption of contaminated fish and other aquatic organisms, particularly large predatory fish species (USEPA, 1997). A major concern related to the health risks of chronic MeHg exposure is the possibility of developmental toxicity in the fetal brain, since MeHg can readily cross the placenta and the blood–brain barrier (Clarkson and Magos, 2006). Prenatal Hg exposure interferes with the growth and migration of neurons and has the potential to cause irreversible damage to the developing central nervous system (Committee on the Toxicological Effects of Methylmercury, 2000). For instance, because of prenatal MeHg exposure from maternal fish consumption, infants might display deficits in subtle neurological endpoints such as IQ deficits, abnormal muscle tone, and decrements in motor function (Committee on the Toxicological Effects of Methylmercury, 2000).
1.3 Sources of Mercury
Both naturally occurring and anthropogenic processes can release mercury into air, water, and soil, and emission into the atmosphere is usually the primary pathway for mercury entering the environment (Camargo, 1993; Berg et al., 2006; Jiang et al., 2006; Bone et al., 2007; Bookman et al., 2008; Streets et al., 2009; Cheng and Hu, 2010). It is estimated that the total annual global input to the atmosphere from all sources (i.e., from natural and anthropogenic emissions) is around 5000–6000 t (Mason et al., 1994; Lamborg et al., 2002; Gray and Hines, 2006). The relative importance of natural versus anthropogenic sources of mercury has not been accurately determined, with the ratio of natural to anthropogenic mercury emissions being reported to be within a wide range (e.g., from 0.8 to 1.8) (Nriagu and Pacyna, 1988; Nriagu, 1989, 1994; Bergan et al., 1999; Gustin et al., 2000; Lin and Tao, 2003; Nriagu and Becker, 2003; Seigneur et al., 2003, 2004; Gbor et al., 2007; Shetty et al., 2008).
1.3.1 Natural Sources of Mercury
There are a number of natural processes that can emit Hg into the atmosphere. These processes may include geologic activities (in particular volcanic and geothermal emissions), volatilization of Hg in marine environments, and emission of Hg from terrestrial environments (including substrates with elevated Hg concentrations and background soils) (Nriagu, 1989, 1993, 1994; Gustin et al., 2000, 2008; Gustin, 2003; Nriagu and Becker, 2003; Gray and Hines, 2006). Owing to the lack of data and the complexity of geological processes (e.g., vast variability spatially and temporally) (Gustin et al., 2000, 2008), it is rather difficult to accurately estimate natural Hg emissions, resulting in high degrees of uncertainties being associated with the reported Hg emissions from natural sources. The annual global Hg emissions from natural sources are estimated to range from 800 to 5800 t, with a middle range from 1800 to 3000 t (Lindberg and Turner, 1977; Nriagu, 1989; Lindberg et al., 1998; Bergan et al., 1999; Pirrone et al., 2001; Seigneur et al., 2001, 2004; Lamborg et al., 2002; Mason and Sheu, 2002; Pacyna and Pacyna, 2002; Pirrone and Mahaffey, 2005; Pacyna et al., 2006; Shetty et al., 2008). Among different natural processes, the global volcanic, geothermal, oceanic, and terrestrial Hg emissions are estimated to be 1–700, ∼60, 800–2600, and 1000–3200 t per year, respectively (Nriagu, 1989; Lindberg et al., 1998, 1999; Bergan et al., 1999; Ferrara et al., 2000; Lamborg et al., 2002; Mason and Sheu, 2002; Nriagu and Becker, 2003; Pyle and Mather, 2003; Seigneur et al., 2004; Fitzgerald et al., 2007b). Gaseous elemental mercury (GEM) is the predominant form (>99%) of Hg from natural emissions, which is different than anthropogenic emissions that may also contain reactive gaseous mercury (RGM) and particulate Hg (PHg) (Stein et al., 1996; Streets et al., 2005; Pacyna et al., 2006). It should be noted that some processes of natural Hg emissions include reemission of Hg previously deposited from the atmosphere by wet and dry processes derived from both anthropogenic and natural sources. For instance, emission from low Hg-containing substrates and background soils is assumed to be predominantly reemission of Hg previously deposited (Gustin et al., 2000; Seigneur et al., 2004; Gustin et al., 2008; Shetty et al., 2008).
1.3.2 Anthropogenic Sources of Mercury
Extensive anthropogenic emission and use of Hg have caused worldwide mercury contamination in many aquatic and terrestrial ecosystems (Lee et al., 2001; Streets et al., 2005, 2009; Hope, 2006; Wu et al., 2006; Zhang and Wong, 2007; Sunderland et al., 2009). Comparisons of contemporary (within the past 20–30 years) measurements and historical records indicate that the total global atmospheric mercury burden has increased by a factor of between 2 and 5 since the beginning of the industrialized period (USEPA, 1997). Although anthropogenic emission of Hg has been reduced in the past three decades, anthropogenic processes are still responsible for a significant proportion of global Hg input to the environment. It has been suggested that, among the 5000–6000 t of Hg that is estimated to be released into the atmosphere each year, about 50% may be from anthropogenic sources (Mason et al., 1994; Lamborg et al., 2002; Gray and Hines, 2006), which agrees with some other studies where the annual global anthropogenic emissions of mercury are estimated to be in the range of 2000–2600 t (Pacyna et al., 2001, 2006; Pirrone et al., 2001; Pacyna and Pacyna, 2002; Pirrone and Mahaffey, 2005). Unlike natural sources, anthropogenic sources can emit different species of Hg including GEM, RGM, and PHg with a distribution of about 50–60% GEM, 30% RGM, and 10% PHg (Streets et al., 2005; Pacyna et al., 2006).
Anthropogenic emissions of mercury can be from point (e.g., incinerators and coal-fired power plants) as well as diffuse (e.g., landfills, sewage sludge amended fields, and mine waste) sources (Nriagu, 1989; Sigel and Sigel, 2005; Malm, 1998; Schroeder and Munthe, 1998; Quemerais et al., 1999; Lee et al., 2001; Horvat, 2002; Gustin, 2003; Nelson, 2007; Feng et al., 2010; Pacyna et al., 2010). Point sources, including combustion, manufacturing, and miscellaneous sources (e.g., dental amalgam), are thought to be the main anthropogenic sources of mercury, accounting for approximately more than 95% of anthropogenic mercury emissions (USEPA, 1997). Combustion sources include burning of fossil fuels (e.g., coal and oil), medical waste incinerators, municipal waste combustors, and sewage sludge incinerators. Fossil fuel combustion can be associated with power generation, industrial and residential heating, and various industrial processes. Combustion processes emit divalent mercury and elemental mercury, in gaseous as well as particulate form, depending on the fuels and materials burned (e.g., coal, oil, municipal waste) and fuel gas cleaning and operating temperature, into the atmosphere (USEPA, 1997; UNEP Chemicals Branch, 2008). Manufacturing sources refer to extensive use (especially in the past and in some undeveloped areas) of mercury compounds in many industrial processes such as gold mining, chlor-alkali production, and paper and pulp manufacturing. Unlike combustion sources, manufacturing processes can release mercurial compounds directly into aquatic and terrestrial environments, in addition to the atmosphere (Lindberg and Turner, 1977; Nriagu et al., 1992; Nriagu, 1994; USEPA, 1997; AMAP/UNEP, 2008; UNEP Chemicals Branch, 2008).
Of the three anthropogenic point sources, combustion generally contributes more than 80% of anthropogenic mercury emissions, although varying from region to region (USEPA, 1997; UNEP Chemicals Branch, 2008). Figure 1.1 illustrates the global inventory of mercury emissions from major anthropogenic sources, as estimated by the United Nations Environmental Programme (UNEP) (AMAP/UNEP, 2008; UNEP Chemicals Branch, 2008). Fossil fuel combustion for power generation and industrial and residential heating contributes about 45% of total global emission (880 t out of 1930 t) (Fig. 1.1). Owing to the enormous amount of coal that is burned, coal burning is the largest single source of anthropogenic emissions of Hg to the atmosphere (AMAP/UNEP, 2008). Waste incineration contributes another significant proportion (about 120 t) of mercury emission, but with a wide range between 50 and 470 t due to lack of reliable estimation data, in particular in countries outside Europe and North America. In addition, fuel combustion in industrial processes, including cement and metal production, can release mercury into the atmosphere. Meanwhile, these industrial processes, in particular, the production of iron and nonferrous metals, can release mercury as it can be present as impurity in ores (AMAP/UNEP, 2008). The data illustrated in Fig. 1.1 for these industrial processes include mercury from fuel combustion and from impurities in ores.
Figure 1.1 Annual global mercury emission (tons) from major anthropogenic sources.
Source: Data are extracted from the UNEP reports (AMAP/UNEP, 2008; UNEP Chemicals Branch, 2008). Fossil fuel combustion refers to burning of coal and other fossil fuels in power plants and commercial and residential heating units. Metal production includes mercury production, but does not include gold mining and production, which is listed separately.
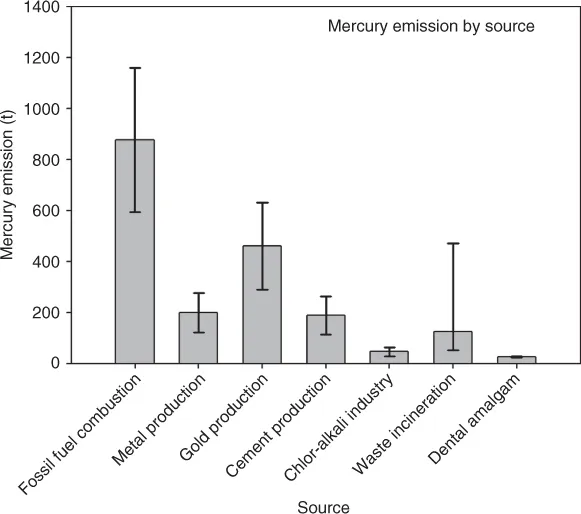
Manufacturing sources mainly include gold mining and chlor-alkali industry. Globally, gold mining and production, primarily artisanal and small-scale gold mining using mercury to extract gold, contribute about 20% of anthropogenic mercury emission, while the fraction for chlor-alkali production is about 3% (Fig. 1.1) (AMAP/UNEP, 2008...
Table of contents
- Cover
- Title Page
- Copyright
- Preface
- Acknowledgment
- Contributors
- Chapter 1: Overview of Mercury in the Environment
- Part I: Analytical Developments
- Part II: Speciation and Transformation
- Part III: Transport and Fate
- Part IV: Bioaccumulation, Toxicity, and Metallomics
- Index