Dougald M. Monroe1 and Maureane Hoffman2
Historical background
Any mechanistic description of blood coagulation should account for a number of simple observations about the blood coagulation process. Blood that is circulating inside the body tends not to clot. However, blood that escapes from the vasculature does clot. This suggests that there is a material outside blood that is necessary for the clotting process. This point was emphasized by the study of Foà and Pellacani who showed that “tissue juice” (filtered saline extract of brain), when injected into the circulation of a rabbit, could cause intravascular thrombus formation [1]. This result was further clarified by Macfarlane and Biggs who showed that blood had all the factors needed to clot (intrinsic factors) but that this process was slow and that clotting was accelerated by the addition of tissue extracts (extrinsic factors) [2].
A clotted mass of blood was called a thrombus. When this thrombus was washed, a material, thrombin, could be eluted that would immediately clot fresh blood. It was further shown that there existed in blood an inactive agent, prothrombin, which could be converted to active thrombin. The agent responsible for this conversion was called thromboplastin (or thrombokinase). Attempts to discover the nature of thromboplastin led to much of our current mechanistic understanding of coagulation.
In 1875, Zahn made the important observation that bleeding from a blood vessel was blocked by a white (not red) thrombus [3]. Bizzozero and Hayem, working separately, studied a colorless corpuscle in blood called a thrombocyte or platelet [4,5]. This cell could be shown to be associated with fibrin and was postulated to be a major component of the white thrombus [4]. It was therefore suggested that there was a platelet thromboplastin that was critical for clotting (in modern usage, platelet procoagulant function is described as such and the term thromboplastin is used to mean the protein tissue factor [TF] which is the coagulation initiator in tissues).
It is known that in some families there is an inherited bleeding tendency (hemophilia). Eagle studied individuals with this disorder and showed that the platelet function in those patients was normal but that there was still a deficiency in prothrombin conversion [6]. This established that there was a plasma component required for clotting in addition to a requirement for platelets. Further studies in patients with different bleeding tendencies established that there are a number of elements that make up the plasma clotting component. Because these factors were discovered by multiple investigators in different parts of the world (and given a different name by each group), a systematic nomenclature was established using Roman numerals [7] (Table 1.1).
Table 1.1 Systematic nomenclature of clotting factors.
|
| I | Fibrinogen |
| II | Prothrombin |
| III | Lipid, platelet surface, or Thromboplastin (not used) |
| IV | Calcium (not used) |
| V | |
| VI | Activated factor V (not used) |
| VII | |
| VIII | Hemophilia A factor |
| IX | Hemophilia B factor |
| X | |
| XI | Hemophilia C factor |
| XII | |
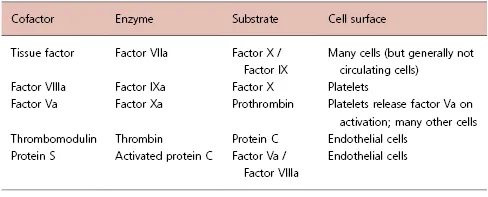
While studies on deficient plasmas had established what the important components were, the mechanisms of action and the interactions between these components were not immediately clear. Early coagulation schemes started from the model of prothrombin being converted to thrombin and visualized all of the circulating coagulation proteins as zymogens that were converted during coagulation into active enzymes [8,9]. Once the proteins involved in coagulation were isolated and their structure and functions were studied, it became clear that coagulation function was organized around a mechanism of an active enzyme being paired with a cofactor [10]. In the absence of the cofactor, the enzyme has limited activity; typically a cofactor will accelerate the activity of a coagulation enzyme as much as 1000-fold [11]. Thus, each step in coagulation is regulated at two levels: 1) activation of the zymogen to an active enzyme and 2) the presence of (and sometimes activation of) the requisite cofactor. Since some cofactors, such as thromboplastin (tissue factor) and thrombomodulin, are integral membrane proteins, the functions of these complexes can be limited to cells and tissues that express the protein (Table 1.2).
Table 1.2 Composition and physiologic location of coagulation complexes.
The coagulation factors show only weak activity in solution, and binding to an appropriate cell surface accelerates their activity up to 1000-fold. This surface binding is dependent on calcium and, therefore, blood can be anticoagulated by the addition of chelating agents such as citrate or EDTA that bind calcium [12]. This chelation does not alter protein properties and can be readily reversed by reintroduction of calcium in excess of the chelating agents. Clinical assays use plasma prepared from blood chelated with citrate to analyze clotting factor function by addition of an appropriate activator and calcium and measuring the time to clot formation.
Localization of the coagulation reactions to a desired surface represents a powerful mechanism for limiting coagulation to surfaces at the site of injury. One component of coagulation factor binding to cells is the phospholipid composition of the outer leaflet of the cell membrane. Phospholipids with acidic head groups, phosphatidic acid (PA) and phosphatidylserine (PS), promote binding of coagulation factors [13]. In addition, phosphatidylserine acts as an allosteric regulator of function and accounts for the ability of PS-containing membranes to enhance coagulation factor activity [14]. While generic phospholipid surfaces can support coagulation reactions (and are used in clinical assays), it is clear that cells, in addition to having appropriate lipid surfaces, have regulatory elements that control the coagulation reactions [15,16].
Functional platelets are required as a surface for hemostasis, and patients with low platelet counts (thrombocytopenia) or platelet function defects (thrombocytopathia) such as Bernard–Soulier syndrome or Glanzmann's thrombasthenia have a bleeding tendency. Circulating platelets, like essentially all cells in blood as well as endothelial cells, have outer membranes with low levels of acidic phospholipids. When platelets adhere at a site of injury the composition of their membrane changes such that acidic phospholipids, including phosphatidylserine, are now expressed on the outer surface of the membranes [17]. This change in surface lipid composition, along with changes in platelet surface proteins and release of procoagulant factors from platelet granules, provides a surface that supports robust coagulation.
Cell-based model of coagulation
In a mild injury, the coagulation process starts with hemostatic platelet aggregates which can be found at the ends of transected blood vessels [18]. Early in the process, these aggregates consist of activated (degranulated) platelets packed together. In time, small amounts of fibrin are deposited between the platelets. At longer times more fibrin becomes associated with the platelet masses. This fibrin extends into the tissues and provides stability to the area of injury [18,19].
In hemophilia A or B, the process is somewhat different [20]. Early in the process, the platelets are loosely associated but are not activated. Even at longer times platelets are only poorly activated and fibrin is not seen between the platelets. The result is that the platelet mass is not stabilized. Whereas normal individuals show extensive fibrin extending into the tissues, in hemophilia a thin layer of fibrin can be seen only at the margins of the wound area and does not extend significantly into the tissues [19,20].
These observations of hemostasis suggest that coagulation can be conceived of as a series of overlapping steps: initiation; amplification; and propagation.
Initiation
Blood coagulation is initiated by an injury to a blood vessel; this injury could be a denudement of some of the endothelium or a break in the vessel. In either case, two processes begin immediately. One process is that platelets quickly adhere to the site of injury. This adherence requires von Willebrand factor which binds to both collagen in the exposed subendothelium and the abundant platelet protein glycoprotein Ib. This adherence brings platelets into contact with collagen which, through the platelet collagen receptor glycoprotein VI, activates platelets [21]. This activation causes changes in platelet surface receptors and leads the platelets to degranulate. Degranulation releases a number of stored proteins including a partially activated form of factor V [22].
The second process that begins with a break in the vasculature is that plasma concentrations of coagulation proteins are brought into the area of injury and presented to extravascular cells. Cells surrounding the vasculature tend to be rich in the protein called tissue factor (thromboplastin); the high concentration of tissue factor around blood vessels has been described as contributing to a hemostatic envelope [23]. At least some of the tissue factor already has factor VII bound [24] and factor VII binds tightly to any free tissue factor. On cells this tissue factor-bound factor VII is rapidly converted to factor VIIa. This conversion can be via cellular proteases, by autoactivation by other factor VIIa molecules, or by factor Xa generated by factor VIIa–tissue factor complexes [25,26].
These factor VIIa–tissue factor complexes catalyze two reactions: activation of factor X and activation of factor IX [27]. The factor Xa that is formed can complex with the partially active factor V released from platelets; this factor Xa–Va complex converts at least some prothrombin to thrombin. Formation of factor Xa also starts the process of regulating coagulation. The inhibitor TFPI (tissue factor pathway inhibitor) can bind to factor Xa and factor VIIa to turn off the factor VIIa–tissue factor complex [28,29]. This inhibition requires factor Xa so that the factor VIIa–tissue factor complex is not turned off until some factor Xa has been formed. Factor Xa in a complex with factor Va is protected from the abundant plasma inhibitor antithrombin, but once released from the complex, factor Xa inhibition by antithrombin is rapid with an expected half-life of about 4 minutes.
Amplification
The initial thrombin formed during the initiation phase is probably not sufficient to provide for robust fibrin formation and hemostasis. However, the throm...