
The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging
- English
- ePUB (mobile friendly)
- Available on iOS & Android
The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging
About this book
Magnetic Resonance Imaging (MRI) is one of the most important tools in clinical diagnostics and biomedical research. The number of MRI scanners operating around the world is estimated to be approximately 20,000, and the development of contrast agents, currently used in about a third of the 50 million clinical MRI examinations performed every year, has largely contributed to this significant achievement.
This completely revised and extended second edition:
- Includes new chapters on targeted, responsive, PARACEST and nanoparticle MRI contrast agents.
- Covers the basic chemistries, MR physics and the most important techniques used by chemists in the characterization of MRI agents from every angle from synthesis to safety considerations.
- Is written for all of those involved in the development and application of contrast agents in MRI.
- Presented in colour, it provides readers with true representation and easy interpretation of the images.
A word from the Authors:
Twelve years after the first edition published, we are convinced that the chemistry of MRI agents has a bright future. By assembling all important information on the design principles and functioning of magnetic resonance imaging probes, this book intends to be a useful tool for both experts and newcomers in the field. We hope that it helps inspire further work in order to create more efficient and specific imaging probes that will allow materializing the dream of seeing even deeper and better inside the living organisms.
Reviews of the First Edition:
"...attempts, for the first time, to review the whole spectrum of involved chemical disciplines in this technique..."—Journal of the American Chemical Society
"...well balanced in its scope and attention to detail...a valuable addition to the library of MR scientists..."—NMR in BiomedicineFrequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Chapter 1
General Principles of MRI
1.1 Introduction
1.2 Theoretical basis of NMR
1.2.1 Short description of NMR
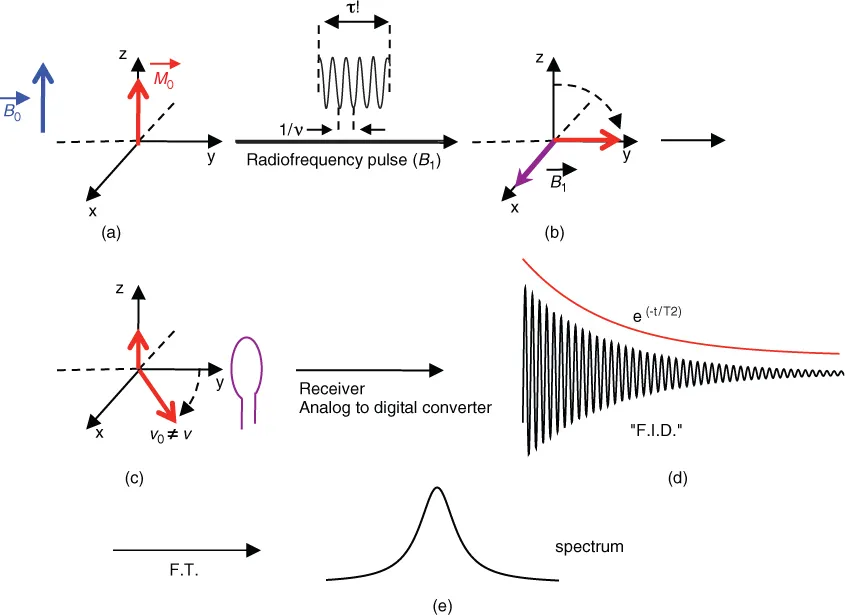
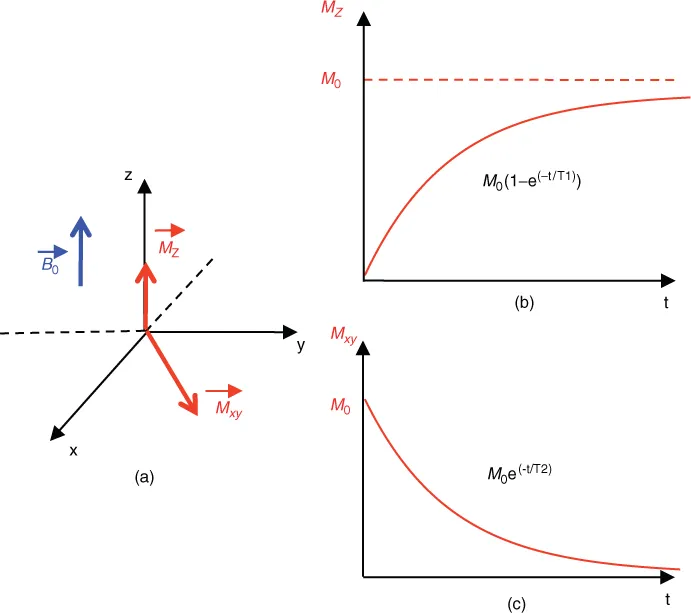
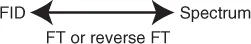
1.2.2 Relaxation times
1.2.3 Saturation transfer
Table of contents
- Cover
- Title Page
- Copyright
- List of Contributors
- Preface
- Chapter 1: General Principles of MRI
- Chapter 2: Relaxivity of Gadolinium(III) Complexes: Theory and Mechanism
- Chapter 3: Synthesis and Characterization of Ligands and their Gadolinium(III) Complexes
- Chapter 4: Stability and Toxicity of Contrast Agents
- Chapter 5: Structure, Dynamics, and Computational Studies of Lanthanide-Based Contrast Agents
- Chapter 6: Electronic Spin Relaxation and Outer-Sphere Dynamics of Gadolinium-Based Contrast Agents
- Chapter 7: Targeted MRI Contrast Agents
- Chapter 8: Responsive Probes
- Chapter 9: Paramagnetic CEST MRI Contrast Agents
- Chapter 10: Superparamagnetic Iron Oxide Nanoparticles for MRI
- Chapter 11: Gd-Containing Nanoparticles as MRI Contrast Agents
- Index