
Evaluation of Enzyme Inhibitors in Drug Discovery
A Guide for Medicinal Chemists and Pharmacologists
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Evaluation of Enzyme Inhibitors in Drug Discovery
A Guide for Medicinal Chemists and Pharmacologists
About this book
Offers essential guidance for discovering and optimizing novel drug therapies
Using detailed examples, Evaluation of Enzyme Inhibitors in Drug Discovery equips researchers with the tools needed to apply the science of enzymology and biochemistry to the discovery, optimization, and preclinical development of drugs that work by inhibiting specific enzyme targets. Readers will applaud this book for its clear and practical presentations, including its expert advice on best practices to follow and pitfalls to avoid.
This Second Edition brings the book thoroughly up to date with the latest research findings and practices. Updates explore additional forms of enzyme inhibition and special treatments for enzymes that act on macromolecular substrates. Readers will also find new discussions detailing the development and application of the concept of drug-target residence time.
Evaluation of Enzyme Inhibitors in Drug Discovery begins by explaining why enzymes are such important drug targets and then examines enzyme reaction mechanisms. The book covers:
- Reversible modes of inhibitor interactions with enzymes
- Assay considerations for compound library screening
- Lead optimization and structure-activity relationships for reversible inhibitors
- Slow binding and tight binding inhibitors
- Drug-target residence time
- Irreversible enzyme inactivators
The book ends with a new chapter exploring the application of quantitative biochemical principles to the pharmacologic evaluation of drug candidates during lead optimization and preclinical development.
The Second Edition of Evaluation of Enzyme Inhibitors in Drug Discovery continues to offer a treatment of enzymology applied to drug discovery that is quantitative and mathematically rigorous. At the same time, the clear and simple presentations demystify the complex science of enzymology, making the book accessible to many fields— from pharmacology to medicinal chemistry to biophysics to clinical medicine.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Key Learning Points
- Enzymes are excellent targets for pharmacological intervention, owing to their essential roles in life processes and pathophysiology.
- The structures of enzyme active sites, and other ligand binding pockets on enzymes, are ideally suited for high-affinity interactions with drug-like inhibitors.
1.1 Enzymes Are Essential for Life
| Compound | Target Enzyme | Clinical Use |
|---|---|---|
| Acetazolamide | Carbonic anhydrase | Glaucoma |
| Acyclovir | Viral DNA polymerase | Herpes |
| Amprenavir, indinavir, nelfinavir, ritonavir, saquinavir | HIV protease | AIDS |
| Allopurinol | Xanthine oxidase | Gout |
| Argatroban | Thrombin | Heart disease |
| Aspirin | Cyclooxygenases | Inflammation, pain, fever |
| Amoxicillin | Penicillin binding proteins | Bacterial infection |
| Captopril, enalapril | Angiotensin converting enzyme | Hypertension |
| Carbidopa | Dopa decarboxylase | Parkinson’s disease |
| Celebrex, Vioxx | Cyclooxygenase-2 | Inflammation |
| CI-1040, PD0325901 | MAP kinase kinase | Cancer |
| Clavulanate | β-Lactamase | Bacterial resistance |
| Digoxin | Sodium, potassium ATPase | Heart disease |
| Efavirenz, nevirapine | HIV reverse transcriptase | AIDS |
| Epristeride, finasteride, dutasteride | Steroid 5α-reductase | Benign prostate hyperplasia, male pattern baldness |
| Fluorouracil | Thymidylate synthase | Cancer |
| Leflunomide | Dihydroorotate | Inflammation |
| Dehydrogenase | ||
| Lovastatin and other statins | HMG-CoA reductase | Cholesterol lowering |
| Methotrexate | Dihydrofolate reductase | Cancer, immunosuppression |
| Nitecapone | Catechol-O-methyltransferase | Parkinson’s disease |
| Norfloxacin | DNA gyrase | Urinary tract infections |
| Omeprazole | H+, K+ ATPase | Peptic ulcers |
| PALA | Aspartate | Cancer |
| Transcarbamoylase | ||
| Sorbinol | Aldose reductase | Diabetic retinopathy |
| Trimethoprim | Bacterial dihydrofolate reductase | Bacterial infections |
| Viagra, Levitra | Phosphodiesterase | Erectile dysfunction |
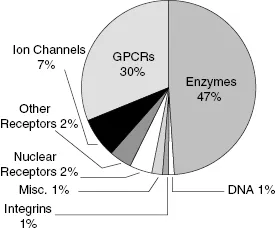
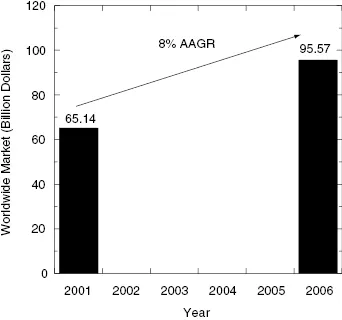
1.2 Enzyme Structure and Catalysis
Table of contents
- Cover
- Title page
- Copyright page
- Dedication
- Foreword to Second Edition
- Preface to Second Edition
- Foreword to First Edition
- Preface to First Edition
- Acknowledgments (From First Edition)
- Epigraph
- CHAPTER 1: Why Enzymes as Drug Targets?
- CHAPTER 2: Enzyme Reaction Mechanisms
- CHAPTER 3: Reversible Modes of Inhibitor Interactions with Enzymes
- CHAPTER 4: Assay Considerations for Compound Library Screening
- CHAPTER 5: Lead Optimization and Structure–Activity Relationships for Reversible Inhibitors
- CHAPTER 6: Slow Binding Inhibitors
- CHAPTER 7: Tight Binding Inhibition
- CHAPTER 8: Drug–Target Residence Time
- CHAPTER 9: Irreversible Enzyme Inactivators
- CHAPTER 10: Quantitative Biochemistry in the Pharmacological Evaluation of Drugs
- APPENDIX 1: Kinetics of Biochemical Reactions
- APPENDIX 2: Derivation of the Enzyme–Ligand Binding Isotherm Equation
- APPENDIX 3: Serial Dilution Schemes
- APPENDIX 4: Relationship between [I]/IC50 and Percentage Inhibition of Enzyme Activity When h = 1
- APPENDIX 5: Propagation of Uncertainties in Experimental Measurements
- APPENDIX 6: Useful Physical Constants at Different Temperatures
- APPENDIX 7: Common Radioactive Isotopes Used in Studies of Enzymes
- APPENDIX 8: Common Prefixes for Units in Biochemistry
- APPENDIX 9: Some Aromatic Ring Systems Commonly Found in Drugs
- APPENDIX 10: Residual Plots
- Index