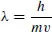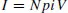![]()
1
Ion Sources
In the ion sources, the analysed samples are ionized prior to analysis in the mass spectrometer. A variety of ionization techniques are used for mass spectrometry. The most important considerations are the internal energy transferred during the ionization process and the physico-chemical properties of the analyte that can be ionized. Some ionization techniques are very energetic and cause extensive fragmentation. Other techniques are softer and only produce ions of the molecular species. Electron ionization, chemical ionization and field ionization are only suitable for gas-phase ionization and thus their use is limited to compounds sufficiently volatile and thermally stable. However, a large number of compounds are thermally labile or do not have sufficient vapour pressure. Molecules of these compounds must be directly extracted from the condensed to the gas phase.
These direct ion sources exist under two types: liquid-phase ion sources and solid-state ion sources. In liquid-phase ion sources the analyte is in solution. This solution is introduced, by nebulization, as droplets into the source where ions are produced at atmospheric pressure and focused into the mass spectrometer through some vacuum pumping stages. Electrospray, atmospheric pressure chemical ionization and atmospheric pressure photoionization sources correspond to this type. In solid-state ion sources, the analyte is in an involatile deposit. It is obtained by various preparation methods which frequently involve the introduction of a matrix that can be either a solid or a viscous fluid. This deposit is then irradiated by energetic particles or photons that desorb ions near the surface of the deposit. These ions can be extracted by an electric field and focused towards the analyser. Matrix-assisted laser desorption, secondary ion mass spectrometry, plasma desorption and field desorption sources all use this strategy to produce ions. Fast atom bombardment uses an involatile liquid matrix.
The ion sources produce ions mainly by ionizing a neutral molecule in the gas phase through electron ejection, electron capture, protonation, deprotonation, adduct formation or by the transfer of a charged species from a condensed phase to the gas phase. Ion production often implies gas-phase ion–molecule reactions. A brief description of such reactions is given at the end of the chapter.
1.1 Electron Ionization
The electron ionization (EI) source, formerly called electron impact, was devised by Dempster and improved by Bleakney [1] and Nier [2]. It is widely used in organic mass spectrometry. This ionization technique works well for many gas-phase molecules but induces extensive fragmentation so that the molecular ions are not always observed.
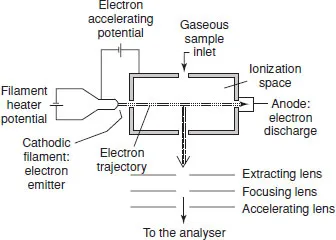
As shown in Figure 1.1, this source consists of a heated filament giving off electrons. The latter are accelerated towards an anode and collide with the gaseous molecules of the analysed sample injected into the source. Gases and samples with high vapour pressure are introduced directly into the source. Liquids and solids are usually heated to increase the vapour pressure for analysis.
Figure 1.1 Diagram of an electron ionization source.
Each electron is associated to a wave whose wavelength λ is given by
where m is its mass, v its velocity and h Planck’s constant. This wavelength is 2.7 Å for a kinetic energy of 20 eV and 1.4 Å for 70 eV. When this wavelength is close to the bond lengths, the wave is disturbed and becomes complex. If one of the frequencies has an energy hv corresponding to a transition in the molecule, an energy transfer that leads to various electronic excitations can occur [3]. When there is enough energy, an electron can be expelled. The electrons do not ‘impact’ molecules. For this reason, it is recommended that the term electron impact must be avoided.
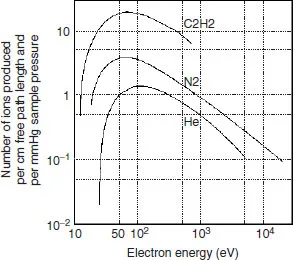
Figure 1.2 displays a typical curve of the number of ions produced by a given electron current, at constant pressure of the sample, when the acceleration potential of the electrons (or their kinetic energy) is varied [4]. At low potentials the energy is lower than the molecule ionization energy. At high potentials, the wavelength becomes very small and molecules become ‘transparent’ to these electrons. In the case of organic molecules, a wide maximum appears around 70 eV. At this level, small changes in the electron energy do not significantly affect the pattern of the spectrum.
On average, one ion is produced for every 1000 molecules entering the source under the usual spectrometer conditions, at 70 eV. Furthermore, between 10 and 20 eV is transferred to the molecules during the ionization process. Since approximately 10 eV is enough to ionize most organic molecules, the excess energy leads to extensive fragmentation. This fragmentation can be useful because it provides structural information for the elucidation of unknown analytes.
At a given acceleration potential and at constant temperature, the number of ions I produced per unit time in a volume V is linked to the pressure p and to the electron current i through the following equation, where N is a constant proportionality coefficient:
Figure 1.2 Number of ions produced as a function of the electron energy. A wide maximum appears around 70 eV.
This equation shows that the sample pressure is directly correlated with the resulting ionic current. This allows such a source to be used in quantitative measurements.
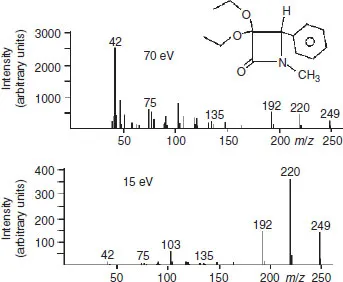
Figure 1.3 displays two EI spectra of the same β-lactam compound, obtained at 70 and 15 eV. Obviously, at lower energy there is less fragmentation. At first glance, the molecular ion is better detected at low energy. However, the absolute intensity, in arbitrary units, proportional to the number of detected ions, is actually lower: about 250 units at 70 eV and 150 units at 15 eV. Thus, the increase in relative intensity, due to the lower fragmentation, is illusory. Actually, there is a general loss of intensity due to the decrease in ionization efficiency at lower electron energy. This will generally be the rule, so that the method is not very useful for better detection of the molecular ion. However, the lowering of the ionization voltage may favour some fragmentation processes.
A modification implies desorbing the sample from a heated rhenium filament near the electronic beam. This method is called desorption electron ionization (DEI).
Under conventional electron ionization conditions, the formation of negative ions is inefficient compared with the formation of positive ions.
1.2 Chemical Ionization
Electron ionization leads to fragmentation of the molecular ion, which sometimes prevents its detection. Chemical ionization (CI) is a technique that produces ions with little excess energy. Thus this technique presents the advantage of yielding a spectrum with less fragmentation in which the molecular species is easily recognized. Consequently, chemical ionization is complementary to electron ionization.
Figure 1.3 Two spectra of β-lactam. While the relative intensity of the molecular ion peak is greater at lower ionization energy, its absolute intensity, as read from the left-hand scale, is actually somewhat reduced.
Chemical ionization [5] consists of producing ions through a collision of the molecule to be analysed with primary ions present in the source. Ion–molecule collisions will thus be induced in a definite part of the source. In order to do so, the local pressure has to be sufficient to allow for frequent collisions. We saw that the mean free path could be calculated from Equation 1 (see the Introduction). At a pressure of approximately 60 Pa, the free path is about 0.1 mm. The source is then devised so as to maintain a local pressure of that magnitude. A solution consists of introducing into the source a small box about 1 cm along its side as is shown in Figure 1.4.
Two lateral holes allow for the crossing of electrons and another hole at the bottom allows the product ions to pass through. Moreover, there is a reagent gas input tube and an opening for the sample intake. The sample is introduced by means of a probe which will close the opening.
This probe carries the sample within a hollow or contains the end part of a capillary coming from a chromatograph or carries a filament on which the sample was deposited. In the last case, we talk about desorption chemical ionization (DCI). The pumping speed is sufficient to maintain a 60 Pa pressure within the box. Outside, the usual pressure in a source, about 10−3 Pa, will be maintained.
Inside the box, the sample pressure will amount to a small fraction of the reagent gas pressure. Thus, an electron entering the box will preferentially ionize the reagent gas molecules through ele...