![]()
PART I
OVERVIEW OF LC-MS BIOANALYSIS
![]()
1
ROLES OF LC-MS BIOANALYSIS IN DRUG DISCOVERY, DEVELOPMENT, AND THERAPEUTIC DRUG MONITORING
STEVE UNGER, WENKUI LI, JIMMY FLARAKOS, AND FRANCIS L.S. TSE
1.1 INTRODUCTION
Bioanalysis is a subdiscipline of analytical chemistry for the quantitative measurement of xenobiotics (chemically synthesized or naturally extracted drug candidates and genetically produced biological molecules and their metabolites or post-translationally modified products) and biotics (macromolecules, proteins, DNA, large molecule drugs, metabolites) in biological systems. Many scientific decisions regarding drug development are dependent upon the accurate quantification of drugs and endogenous components in biological samples. Unlike its sister subdisciplines of analytical chemistry such as drug substance and drug product analysis, one very unique feature of contemporary bioanalysis is that its measurement target is always at very low concentration levels, typically at low ng/ml concentration range and even at pg/ml for highly potent medicines. It is this very low concentration, compounded by coexisting endogenous or exogenous compounds with similar chemical structures to the target analytes at a much higher concentration (typically at μg/ml to mg/ml range), that challenges bioanalytical scientists to accurately and definitively measure the analytes of interest.
Since its commercial introduction in the 1980s, liquid chromatography–mass spectrometry (LC-MS), or much more predominantly, tandem mass spectrometry (LC-MS/MS) has rapidly become standard instrumentation in any well-equipped bioanalytical laboratory. LC-MS is a combination of the physicochemical separation capabilities of liquid chromatography (LC) and the mass (MS or MS/MS) separation/detection capabilities of mass spectrometry. In LC-MS bioanalysis, assay selectivity can be readily achieved by three stages of separation of the analyte(s) of interest from unwanted components in the biological matrix: (1) sample extraction (protein precipitation, liquid–liquid extraction, solid-phase extraction, etc.), (2) column chromatography, and (3) tandem mass spectrometric detection in selected reaction monitoring (SRM) or multiple reaction monitoring (MRM) mode. Nevertheless, many factors, including matrix effect, ion suppression, and in-source breakdown of labile metabolites, can compromise the reliability of a LC-MS bioanalytical assay. These factors should be carefully evaluated during method development.
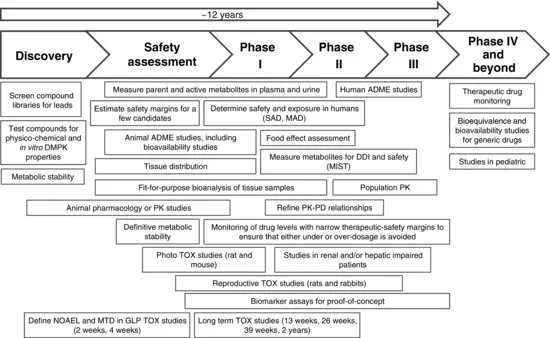
The focus of LC-MS bioanalysis in the pharmaceutical industry is to provide a quantitative measurement of the active drug and/or its metabolite(s) for the accurate assessment of pharmacokinetics, toxicokinetics, bioequivalence (BE), and exposure–response (pharmacokinetics/ pharmacodynamics) relationships (Figure 1.1). The quality of these studies, which are often used to support regulatory filings and other evaluations, is directly related to the conduct of the underlying bioanalysis. Therefore, the application of best practices in bioanalytical method development, validation, and associated sample analysis is key to an effective discovery and development program leading to the successful registration and commercialization of a drug product.
1.2 LC-MS BIOANALYSIS IN DRUG DISCOVERY
Before the introduction of combinatorial chemistry, many drug candidates came from natural products where an active compound was isolated and its chemical structure was characterized using NMR, MS, IR, and derivatization or selective degradation chemistry. Screening entailed an assessment of bioactivity and physicochemical data compared to known databases. High-resolution mass spectrometry played a critical role allowing molecular formula searches from accurate mass data. Similarly, spectral databases allowed positive confirmation or class assessments. This process helped to ensure that novel compounds were selected. Since the introduction of combinatorial chemistry 20 years ago, the analyst's role in early drug discovery has shifted to the development of highly efficient LC-MS analytical methods to support quantitative analysis. The drug discovery process begins with compound library development and ends with the selection of preclinical drug candidates for preclinical safety assessment. LC-MS bioanalysis plays an important role throughout this process.
1.2.1 Structure-Activity Relationships from High-Throughput Screening
High-throughput LC-MS assays can be employed for the determination of solubility, membrane permeability or transport, protein binding, and chemical and metabolic stability for a large number of compounds that have been identified as “hits” (Janiszewski et al., 2008). Thousands of compounds per year go through some or all of these screening procedures. The in vitro studies validate in silico assessments performed prior to synthesis and select compounds for moving forward in development.
1.2.2 Structure—PK-PD Relationships
Selected compounds from high-throughput screening are subsequently evaluated in pharmacology models for efficacy. Provided the targeted biochemistry is applicable to LC-MS analysis, high-throughput screening of potential biomarkers can be performed in pharmacology studies via either a targeted pathway or a metabolomics approach. If successful, discovery biomarkers may be useful in preclinical and clinical studies. Simple examples include steroid biomarkers such as testosterone or dihydrotestosterone for 5-α-reductase inhibitors or estrogen for selective estrogen receptor modulators.
Integration of drug metabolism and pharmacokinetics (DMPK), pharmacology, and biology studies in drug discovery can greatly accelerate an understanding of the pharmacokinetic–pharmacodynamic (PK-PD) relationships of lead compounds. The minimum effective dose observed in the pharmacology model is validated from knowledge of drug and active metabolite levels at the target site and compared with in vitro efficacy. Compounds known to have in vitro potency but are devoid of in vivo activity are suspected of having poor bioavailability (BA) or other DMPK properties (transport to target site, rapid clearance, etc.). Alternatively, compounds with an unanticipated high in vivo activity may have superior access to the site of action or form active metabolites.
LC-MS has a fundamental role in the success of many of these discovery studies. An appropriately designed, early in vitro study can determine intrinsic clearance in multiple species. In vitro assessments have improved our ability to predict systemic clearance using intrinsic clearance. However, predicting volume of distribution and tissue concentrations is far more difficult. Combinatorial approaches such as cassette dosing or coadministration of many compounds is one means of quickly assessing penetration into target sites. Typically, ∼20 compounds are coadministered, but as many as 100 have been attempted (Berman et al., 1997). The specificity of MS detection allows one to simultaneously measure numerous compounds in biofluids and tissues and rapidly screen drug candidates for their ability to penetrate into the site of action (Wu et al., 2000).
1.2.3 Candidate Selection
Within a therapeutic program, a limited number of compounds may be investigated in greater detail as possible preclinical drug candidates. These include assessments at various doses in the rodent and nonrodent toxicology species. Defining the systemic and local exposures, refining PK-PD models and exploring dose-proportionality are among the objectives of this phase. Studies with both single and multiple ascending doses may be undertaken in an effort to assess accumulation, induction and toxicity. Whereas a “generic” LC-MS assay may suffice in supporting these non-GLP assessments of drug properties, one needs to be aware of the potential pitfalls, including stability of parent and metabolites and matrix effects from unknown metabolites, endogenous components, and dosing vehicles such as polyethylene glycols, a frequently used formulation for IV dosage.
As a drug candidate progresses further, translational medicine often will define biomarkers from pharmacology or metabolomics studies that can be used in clinical trials. Over the past 15 years, there has been considerable progress in the use of LC-MS to measure small biochemicals and peptides. The ability to use biomarkers as a surrogate endpoint and to ensure a reliable PK-PD relationship is a common strategy for most drug development programs.
1.3 LC-MS BIOANALYSIS IN PRECLINICAL DEVELOPMENT OF DRUGS
1.3.1 Toxicokinetics
Drug safety assessment studies regulated under good laboratory practice (GLP) are an important part of the preclinical development activities. In a typical toxicology study, toxicokinetic evaluation is performed in order to ascertain adequate drug exposure in the study animals. To support bioanalysis of toxicokinetic samples from the GLP studies, generic LC-MS methods used during drug discovery may no longer be suitable. Modification of the generic method or redevelopment of the respective method is often needed, followed by full assay validation according to the current regulatory guidance and industrial practices (EMA, 2011; FDA 2001; Viswanathan et al., 2007). These requirements are implemented to ensure adequate sensitivity, selectivity, accuracy, precision, reproducibility, and a number of other performance related criteria for a given method.
Preclinical toxicity studies typically employ a broad dose range that could result in a wide range of circulating concentrations of the test compound. Test samples containing analyte levels exceeding the upper limit of quantification (ULOQ) need to be diluted, a step that can sometimes introduce errors. On the other hand, the lower limit of quantification (LLOQ) must be established so that the assay is sensitive enough to measure trough levels from the lowest dose, yet not too sensitive that background noise (false positives) in specimens collected from control animals is detected. A useful rule-of-thumb is to set the LLOQ at ca. 5% of the anticipated peak concentration following the low dose, which should allow accurate analyte measurement for approximately four half-lives.
Different strains of rats such as Sprague Dawley, Wistar Hannover, and Fischer are used in toxicology studies. The LC-MS assay method should be validated using the matrix from the same strain. The beagle dog is generally the default nonrodent species. Nonhuman primates, such as cynomolgus, rhesus, or marmoset monkeys, are occasionally used. The most common use of nonhuman primates is when assessing immunogenicity of large molecule drugs or when the metabolic profiles of dogs differ significantly from human. Drug metabolizing enzymes, such as aldehyde oxidase, can have pronounced differences across species. Matching metabolic profiles to human assures good safety coverage for all metabolites. When metabolism differs across species, metabolism-mediated toxicity can result in sensitivity within one species relative to others. For this reason, there may be a need to measure metabolites in GLP preclinical studies. Although metabolite measurement in those toxicokinetic (TK) samples might be exempt from full GLP compliance due to various reasons, for example, absence of purity certification of reference metabolites and lack of full validation of the intended LC-MS assays, care must be taken to ensure the integrity of the results generated. Often, an assay separate from the parent measurement may be set up for the occasional metabolite quantification. New guidance requires that steady-state exposures of significant metabolites in all species are obtained (Anderson et al., 2010). Non-GLP or tiered assays allow these decisions to be made without extensive validation of multiple assays (Viswanathan et al., 2007).
In parallel with clinical drug development is the continued testing of the compound in animal toxicology studies. This includes extending the safety in primary tox...