
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Chemical Process Technology
About this book
With a focus on actual industrial processes, e.g. the production of light alkenes, synthesis gas, fine chemicals, polyethene, it encourages the reader to think "out of the box" and invent and develop novel unit operations and processes. Reflecting today's emphasis on sustainability, this edition contains new coverage of biomass as an alternative to fossil fuels, and process intensification.
The second edition includes:
- New chapters on Process Intensification and Processes for the Conversion of Biomass
- Updated and expanded chapters throughout with 35% new material overall
- Text boxes containing case studies and examples from various different industries, e.g. synthesis loop designs, Sasol I Plant, Kaminsky catalysts, production of Ibuprofen, click chemistry, ammonia synthesis, fluid catalytic cracking
- Questions throughout to stimulate debate and keep students awake!
- Richly illustrated chapters with improved figures and flow diagrams
Chemical Process Technology, Second Edition is a comprehensive introduction, linking the fundamental theory and concepts to the applied nature of the subject. It will be invaluable to students of chemical engineering, biotechnology and industrial chemistry, as well as practising chemical engineers.
From reviews of the first edition:
"The authors have blended process technology, chemistry and thermodynamics in an elegant manner… Overall this is a welcome addition to books on chemical technology." – The Chemist
"Impressively wide-ranging and comprehensive… an excellent textbook for students, with a combination of fundamental knowledge and technology." – Chemistry in Britain (now Chemistry World)
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
| Scale | Discipline |
| Scale independent | Chemistry, biology, physics, mathematics |
| Thermodynamics | |
| Physical transport phenomena | |
| Micro/nanolevel | Kinetics |
| Catalysis on a molecular level | |
| Interface chemistry | |
| Microbiology | |
| Particle technology | |
| Mesolevel | Reactor technology |
| Unit operations | |
| Macrolevel | Process technology and process development |
| Process integration and design | |
| Process control and operation |
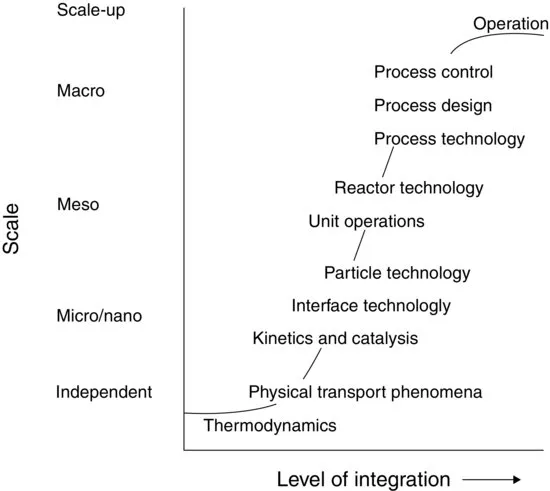
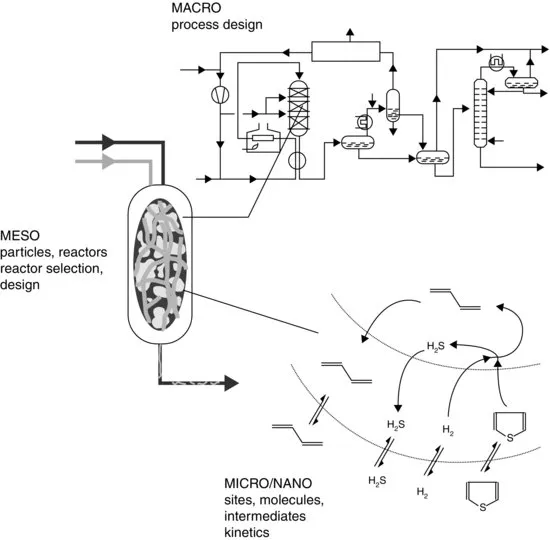
Table of contents
- Cover
- Title Page
- Copyright
- Preface
- Chapter 1: Introduction
- Chapter 2: The Chemical Industry
- Chapter 3: Processes in the Oil Refinery
- Chapter 4: Production of Light Alkenes
- Chapter 5: Production of Synthesis Gas
- Chapter 6: Bulk Chemicals and Synthetic Fuels Derived from Synthesis Gas
- Chapter 7: Processes for the Conversion of Biomass
- Chapter 8: Inorganic Bulk Chemicals
- Chapter 9: Homogeneous Transition Metal Catalysis in the Production of Bulk Chemicals
- Chapter 10: Heterogeneous Catalysis – Concepts and Examples
- Chapter 11: Production of Polymers − Polyethene
- Chapter 12: Production of Fine Chemicals
- Chapter 13: Biotechnology
- Chapter 14: Process Intensification
- Chapter 15: Process Development
- Appendix A: Chemical Industry − Figures
- Appendix B: Main Symbols Used in Flow Schemes
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app