
The Molecular Biology of Cancer
A Bridge from Bench to Bedside
- English
- ePUB (mobile friendly)
- Available on iOS & Android
The Molecular Biology of Cancer
A Bridge from Bench to Bedside
About this book
The Molecular Biology of Cancer, Stella Pelengaris & Michael Khan
This capturing, comprehensive text, extensively revised and updated for its second edition, provides a detailed overview of the molecular mechanisms underpinning the development of cancer and its treatment.
"Bench to Bedside": A key strength of this book that sets it apart from general cancer biology references is the interweaving of all aspects of cancer biology from the causes, development and diagnosis through to the treatment and care of cancer patients – essential for providing a broader view of cancer and its impact.
The highly readable presentation of a complex field, written by an international panel of researchers, specialists and practitioners, would provide an excellent text for graduate and undergraduate courses in the biology of cancer, medical students and qualified practitioners in the field preparing for higher exams, and for researchers and teachers in the field.
For the teaching of cancer biology, special features have been included to facilitate this use: bullet points at the beginning of each chapter explaining key concepts and controversial areas; each chapter builds on concepts learned in previous chapters, with a list of key outstanding questions remaining in the field, suggestions for further reading, and questions for student review. All chapters contain text boxes that provide additional and relevant information.
Key highlights are listed below:
- An overview of the cancer cell and important new concepts.
- Selected human cancers: lung, breast, colorectal, prostate, renal, skin, cervix, and hematological malignancies.
- Key cellular processes in cancer biology including (a) traditionally important areas such as cell cycle control, growth regulation, oncogenes and tumour suppressors apoptosis, as well as (b) more highly topical areas of apoptosis, telomeres, DNA damage and repair, cell adhesion, angiogenesis, immunity, epigenetics, and the proteasome.
- Clinical oncology: In-depth coverage of important concepts such as screening, risk of cancer and prevention, diagnoses, managing cancer patients from start to palliative care and end-of-life pathways.
- Chapters highlighting the direct links between cancer research and clinical applications.
- New coverage on how cancer drugs are actually used in specific cancer patients, and how therapies are developed and tested.
- Systems Biology and cutting edge research areas covered such as RNA interference (RNAi).
- Each chapter includes key points, chapter summaries, text boxes, and topical references for added comprehension and review.
- Quotations have been used in each chapter to introduce basic concepts in an entertaining way.
- Supported by a dedicated website at http://www.blackwellpublishing.com/pelengaris
We should list the great reviews we got for first edition which are on the back of the 2nd edition:
"A capturing, comprehensive, clearly written and absolutely accurate introduction into cancer biology…..This book deserves great praise for the readable presentation of this complex field….the true synthesis of bench and bedside approaches is marvelously achieved." Christian Schmidt, Molecular Cell
"Chapters address the issues of cancer diagnosis, treatment, and patient care and set the book apart from general molecular biology references….This book is applicable to both graduate and undergraduate students, and in the context of a research laboratory, this book would be an excellent resource as a reference guide for scientists at all levels." V.Emuss, Institute of Cancer Research, London.
Also, from the first edition:
"Pelengaris, Khan, and the contributing authors are to be applauded. The Molecular Biology of Cancer is a comprehensive and readable presentation of the many faces of cancer from molecular mechanisms to clinical therapies and diagnostics. This book will be welcomed by neophyte students, established scientists in other fields, and curious physicians." -Dean Felsher, Stanford University
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
- Cancer is a genetic disease characterized by the emergence of deranged versions of normal cells, born out of aberrant molecular biology.
- Cancer is the malign byproduct of an ensemble performance in which mutations in the DNA and altered gene expression are enacted against a background of conniving environmental factors such as carcinogens and chronic inflammation.
- A large number of factors are adduced to explain the genesis of cancer, including the twin pillars of incitement of primeval urges and the emancipation from normal restraining forces. Together, these produce untrammeled cell-cycle progression.
- Studies of rare familial “monogenic” cancer syndromes have had a major impact on our fundamental understanding of cell biology, but most cancers do not result from inheritance of single, potent, cancer-causing mutations.
- Instead, they are “sporadic,” with cancer-causing gene mutations arising in adult somatic cells.
- Hereditary factors may, however, exert weak and subtle influences on the risk of development and subsequently the behavior of most if not all so-called “sporadic tumors,” through a complex interplay between multiple, largely unknown polymorphic alleles, some of which may only be disadvantageous if the individual is exposed to particular environmental carcinogenic factors, such as tobacco smoke.
- In general, factors that cause mutations and those that increase cell replication can combine to cause cancer, which may explain the powerful role of chronic inflammation in the causality of many carcinomas.
- Cancer is a clonal disease arising by the multistep accumulation of genetic or epigenetic changes in tumor suppressor genes, oncogenes, and “caretaker” genes that favor expansion of the new clone over the old in a process akin to Darwinian evolution.
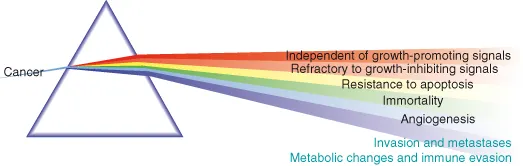
- Natural selection will favor expansion of clones with acquired characteristics advantageous to the cancer cells, often referred to as the “hallmark” features of cancer (Fig. 1.1), which have been famously distilled by Robert A. Weinberg and Douglas Hanahan as:
- Recently, the “Warburg effect,” a metabolic switch towards increasing ATP production by glycolysis, along with evasion or subversion of the immune system have been championed as the seventh and eighth hallmark features, respectively.
- RB and TP53, the doyens of tumor suppressor proteins, can arrest the cell cycle or trigger apoptosis in response to assorted cellular stresses, activation of DNA damage checkpoints, or during attempted oncogenic hijacking of cell-cycle control.
- An intriguing question is exactly how a tumor cell with DNA damage retains so many varied options in the face of TP53 activation? Thus, TP53 can mitigate cell death and inspire DNA repair but, in complete contrast, if repairs fail it might drive a cell to celibacy or suicide.
- Put simply, with respect to replication, a cell with irreparably damaged DNA has to either kick the habit or kick the bucket.
- Not surprisingly, therefore, loss of tumor suppressors is a prerequisite for tumorigenesis. Although there is some overlap, broadly speaking, cancer cells without TP53 can survive an alarming rate of mutation, whereas absence of the other archetypal tumor suppressor, RB, represents a fountain of youth for the otherwise rapidly senescing cancer cell.
- When and where, in the life history of a cancer, do the genetic changes required for metastases occur? There is no satisfactory answer to date. Natural selection does not really provide an explanation as to why a clone of cancer cells with metastatic capabilities would be selected for in the primary tumor, unless the causal mutations first and foremost also provide a growth advantage. It is possible that potential metastatic behavior is serendipitously acquired early in tumorigenesis as a byproduct of mutations promoting growth of the ancestral primary tumor (supported by some gene expression profiling studies of whole tumors). Alternatively, it may be that mutations in specific metastases-suppressing genes that do not confer a growth advantage to the primary occur at a later stage, possibly once cancer cells have begun circulating.
- Recent intriguing questions have been posed regarding the ongoing evolution of cancer cells in primary and secondary tumors. Recent findings suggest that following an initial shared origin, clones with metastatic capabilities emerge in the primary. Once ensconced within a new secondary environment, the metastatic alumni follow a parallel and distinct evolutionary path that may intriguingly begin while still in transit.
- Cancers are complex and heterogeneous, comprising a series of genetically differing populations (clones) of cancer cells. In fact, the dramatis personae of cancer includes the cancer cells-elect, the profligate parents, and a number of libertine relatives of dubious provenance.
- Moreover, the whole ensemble is supported by a strong supporting cast of both collaborating and insurgent noncancer cells that together constitute the cancer microenvironment.
- The cell of origin for any given cancer – be it stem cells that partially differentiate or differentiated cells that partially dedifferentiate, continues to offer opportunities for spirited debate.
- Tumors are not egalitarian societies. Rather they are in most cases oligarchies run by a malign minority of so-called cancer stem cells (CSCs). Part gang master and part queen bee, CSCs lie embedded within a large cast of bit part players. CSCs were first described in hematological malignancies, where they are strongly implicated in maintaining the malignant phenotype. More recently, CSCs have been identified in solid tumors and may be responsible for invasive behaviors, treatment resistance, and recurrence. By implication, these cancer oligarchs are the target of the original cancer-causing mutations, suggesting that in the case of a tumor the fish rots from the head.
- CSCs share many properties and molecular markers with normal stem cells, but this does not constitute proof of paternity. Under the influence of relevant mutations, including those that provoke epithelial–mesenchymal transition (EMT), normal cells can have “stemness” thrust upon them.
- This departs from the more traditional view of indefatigable clonal competition; dog eats dog, the strongest prevails with the extinction of the weakest – aut Caesar aut nihil.
- The cancer microenvironment, including the inflammatory milieu and the tissue stroma (connective tissue, fatty tissue, blood vessels, and lymphatics), represents an alma mater for cancer cells, which by encouraging EMT can help to generate CSCs and support the success of tumorigenesis.
- The greater recognition of the portentous events unfolding within the purlieus of the tumor peripheries during tumorigenesis has already yielded dividends. Thus, the stroma plays society hostess to a prohibition-free orgy of concupiscent cancer cells, egged on by a small faction of attendant immunocytes and under the averted gaze of the rest.
- Remarkably, it now transpires that cancer-contributing mutations are no longer the sole preserve of cancer cells themselves. In fact, mutations in stromal cells may allow them to more effectively mentor cancer cells towards the achievement of their six or eight hallmark milestones.
- The molecular profile of a tumor constitutes a manifesto, within which its future behavior is adumbrated and from which its weaknesses might be divined. Moreover, seminal parts of this manifesto achieve remarkably widespread circulation. Therefore, for diagnosis it may be unnecessary to directly remove tumor tissue, because cells, proteins, and nucleic acids derived from it are continually being shed into more readily accessible body fluids.
- The search for clinically useful molecular biomarkers represents one of the most promising areas of cancer research. Many biomarkers are already in routine clinical practice, where they assist in disease monitoring and in treatment selection.
- However, biomarkers have, as yet, not helped us to paint more accurate portraits of tumors. Unfortunately, in most cases they fail to unambiguously identify their subjects. There is no “Habsburg lip” for cancers.
- In fact, biomarkers have had limited impact on screening the general population for most cancers.
- Given the increasing number of therapeutics in our arsenal against cancer, great efforts are being made to find biomarkers that may help select appropriate treatments for individual patients.
- Cancers may be cured by surgery, but only if the entire tumor is accessible and no cells have spread to other sites. Modern approaches to cancer drug development are increasingly moving away from traditional chemotherapeutic agents which paralyze cell division or cause DNA damage and instead are aimed at targeting specific cancer-relevant proteins, such as oncogenic tyrosine kinases.
- Oncogene addiction, the process by which cancer cells become critically reliant on the mutant signaling molecules, offers the potential of both effective and minimally toxic agents directed against such proteins. A potential realized by pioneering therapeutic successes, such as imatinib, used to such good effect to target the abnormal BCR–ABL fusion protein in chronic myeloid leukemia.
- However, use of these agents is in most cases severely limited by acquired or, on occasion, inherent resistance of cancer cells to the treatment. It is hoped that understanding the resistance mechanisms involved will allow rational development of combinations of targeted agents in the future, though further mutations may render even these ineffective over time. One could easily be forgiven for likening these efforts to cure cancers by drug therapy with the task set before Sysiphus.
- However, we may yet keep the boulder from rolling down the hill. Knowledge is power and by exploiting the potential of treatment biomarkers we may gain an edge over cancer. Thus, we can assess whether a given cancer will respond to particular drugs as exemplified by the presence of estrogen or progesterone receptors and mutant NEU in breast cancer, or may conversely suggest a response to be unlikely as in the presence of KRAS in colorectal cancer. Armed with enough of these biomarkers there is reason to suppose that the goal of individualized medicine and tailored therapy may soon be within reach.
Introduction
Table of contents
- Cover
- Title page
- Copyright page
- Contributors
- Preface to the Second Edition
- Reviews of the First Edition
- Acknowledgments
- Dedication
- About the Companion Website
- Introduction
- 1 Overview of Cancer Biology
- 2 The Burden of Cancer
- 3 Nature and Nurture in Oncogenesis
- 4 DNA Replication and the Cell Cycle
- 5 Growth Signaling Pathways and the New Era of Targeted Treatment of Cancer
- 6 Oncogenes
- 7 Tumor Suppressors
- 8 Cell Death
- 9 Senescence, Telomeres, and Cancer Stem Cells
- 10 Genetic Instability, Chromosomes, and Repair
- 11 There Is More to Cancer than Genetics: Regulation of Gene and Protein Expression by Epigenetic Factors, Small Regulatory RNAs, and Protein Stability
- 12 Cell Adhesion in Cancer
- 13 Tumor Immunity and Immunotherapy
- 14 Tumor Angiogenesis
- 15 Cancer Chemistry: Designing New Drugs for Cancer Treatment
- 16 Biologically Targeted Agents from Bench to Bedside
- 17 The Diagnosis of Cancer
- 18 Treatment of Cancer: Chemotherapy and Radiotherapy
- 19 Caring for the Cancer Patient
- 20 Systems Biology of Cancer
- Glossary
- Answers to Questions
- Index