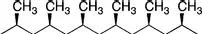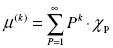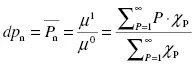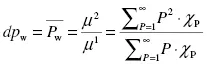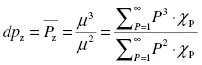![]()
SECTION 1
OVERVIEW OF POLYMERIZATION REACTIONS AND KINETICS
![]()
1
FREE RADICAL AND CONDENSATION POLYMERIZATIONS
MATTHEW KADE AND MATTHEW TIRRELL
1.1 INTRODUCTION
Polymers are macromolecules composed of many monomeric repeat units and they can be synthetic or naturally occurring. While nature has long utilized polymers (DNA, proteins, starch, etc.) as part of life’s machinery, the history of synthetic polymers is barely 100 years old. In this sense, man-made macromolecules have made incredible progress in the past century. While synthetic polymers still lag behind natural polymers in many areas of performance, they excel in many others; it is the unique properties shared by synthetic and natural macromolecules alike that have driven the explosion of polymer use in human civilization. It was Herman Staudinger who first reported that polymers were in fact many monomeric units connected by covalent bonds. Only later we learned that the various noncovalent interactions (i.e., entanglements, attractive or repulsive forces, multivalency) between these large molecules are what give them the outstanding physical properties that have led to their emergence.
In recent years, the uses of synthetic polymers have expanded from making simple objects to much more complex applications such as targeted drug delivery systems and flexible solar cells. In any case, the application for the polymer is driven by its physical and chemical properties, notably bulk properties such as tensile strength, elasticity, and clarity. The structure of the monomer largely determines the chemical properties of the polymer, as well as other important measurable quantities, such as the glass transition temperature, crystallinity, and solubility. While some important determinants of properties, such as crystallinity, can be affected by polymer processing, it is the polymerization itself that determines other critical variables such as the molecular weight, polydispersity, chain topology, and tacticity. The importance of these variables cannot be overstated. For example, a low-molecular-weight stereo-irregular polypropylene will behave nothing like a high-molecular-weight stereo-regular version of the same polymer. Thus, it is easy to see the critical importance the polymerization has in determining the properties and therefore the potential applications of synthetic polymers. It is therefore essential to understand the polymerization mechanisms, the balance between thermodynamics and kinetics, and the effect that exogenous factors (i.e., temperature, solvent, and pressure) can have on both.
1.1.1 Structural Features of Polymer Backbone
1.1.1.1 Tacticity
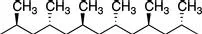
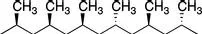
Tacticity is a measure of the stereochemical configuration of adjacent stereocenters along the polymer backbone. It can be an important determinant of polymer properties because long-range microscopic order (i.e., crystallinity) is difficult to attain if there is short-range molecular disorder. Changes in tacticity can affect the melting point, degree of crystallinity, mechanical properties, and solubility of a given polymer. Tacticity is particularly important for a, a′-substituted ethylene monomers (e.g., propylene, styrene, methyl methacrylate). For a polymer to have tacticity, it is a requirement that a does not equal a′ because otherwise the carbon in question would not be a stereocenter. The tacticity is determined during the polymerization and is unaffected by the bond rotations that occur for chains in solution. The simplest way to visually represent tacticity is to use a Natta projection, as shown in Figures 1.1–1.3 using poly (propylene) as a representative example.
An isotactic chain is one in which all of the substituents lie in the same plane (i.e., they have the same stereochemistry). Isotactic polymers are typically semicrystalline and often adopt a helical configuration. Polypropylene made by Ziegler–Natta catalysis is an isotactic polymer.
A syndiotactic chain is the one where the stereochemical configuration between adjacent stereocenters alternates.
An atactic chain lacks any stereochemical order along the chain, which leads to completely amorphous polymers.
1.1.1.2 Composition
Copolymer composition influences a number of quantities, including the glass transition temperature. One commercially relevant example of this effect is with Eastman’s copolymer Tritan™, which has been replacing polycarbonate in a number of applications due to concerns over bisphenol-A’s (BPA’s) health effects. Tritan™ can be considered poly(ethylene terephthalate) (PET), where a percentage of the ethylene glycol is replaced by 2,2,4,4-tetra-methyl-1,3-cyclobutane diol (TMCBDO). In the case of beverage containers, Tg must be greater than 100 °C so they can be safely cleaned in a dishwasher or autoclave. The Tg of Tritan is engineered to be ~110 °C by tuning the relative incorporation of the ethylene glycol (low Tg) and TMCBDO (Tg-increasing) diol monomers.
Altering the glass transition temperature is by no means the only reason to include comonomers in a polymerization. In designing copolymers with specialized applications, comonomers can be included for specific functions, or as sites for further functionalization or initiation of a secondary polymerization (e.g., to make graft copolymers in a graft-from approach). In more broadly used commercial polymers, comonomers can be included to alter different properties, including swelling in particular solvents, stability, viscosity, or to induce self-assembly (e.g., styrene-butadiene-styrene rubbers where styrene domains within the butadiene matrix provide mechanical integrity). While block copolymers produced in sequential polymerizations are not confronted with the problem of unequal reactivity, monomers often have different reactivities within a polymerization. Such discrepancies lead to differences between the composition of monomer feed and the composition of the final polymer.
1.1.1.3 Sequence
The difference in reactivity between comonomers affects the composition and also alters the placement of the monomer units along the chain. In the case of living polymerization, sequential monomer addition leads to the formation of block copolymers. However, when a random copolymer is targeted, reactivity differences can lead to nonrandom distribution of monomer units. If the incorporation of a comonomer B is intended to disrupt crystallinity of poly(A), uninterrupted sequences of A can lead to domains of crystallinity. For example, block copolymers of ethylene–propylene are highly crystalline, while random copolymers are completely amorphous.
1.1.1.4 Regioselectivity
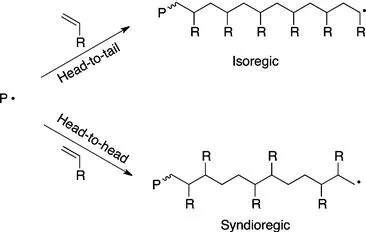
The issue of regioselectivity is most relevant here to vinyl monomers undergoing free radical polymerization, but also applies to other polymerization mechanisms discussed (particularly the synthesis of conducting polymers, which often entails the use of monomers bearing alkyl chains designed to improve solubility). The example of 1-substituted ethylene derivatives (e.g., styrene) is shown in Scheme 1.1. When a propagating chain adds a monomer unit, the radical can add to either C1 or C2. If each successive addition occurs in the same fashion, the result is an isoregic chain, typically referred to as a head-to-tail arrangement.
The alternate configuration is achieved when each successive monomer addition alternates between C1 and C2 additions, giving a syndioregic chain, commonly called a head-to-head arrangement. For free radical polymerizations, isoregic addition is overwhelmingly favored. This is due jointly to resonance and/or inductive stabilization of the resulting radical, which favors head-to-tail addition, and steric constriction around the R group, which discourages head-to-head addition.
1.1.2 The Chain Length Distribution
It is evident that the molecular weight of a polymer chain determines important properties such as viscosity and mechanical strength. Because synthetic polymers do not have a single chain length i and are instead polydisperse, any measure of molecular weight is an average. The chain length distribution is typically characterized by the first three moments of the distribution, where the kth moment is described as follows:
where P is the length of an individual polymer chain and χP is the number of chains of length P.
The weighted degrees of polymerization are defined as the ratio of successive moments, as seen in Equations 1.2 through 1.4:
The number-average degree of polymeriza...