
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Reaction Mechanisms in Organic Synthesis
About this book
Reaction Mechanisms in Organic Synthesis is written from the point of view of the synthetic organic chemist, enabling students and researchers to understand and expand on reactions covered in foundation courses, and to apply them in a practical context by designing syntheses. As a further aid to the practical research student, the content is organized according to the conditions under which a reaction is executed rather than by the types of mechanisms. Particular emphasis is placed on controlling stereospecificity and regiospecificity.
Topics covered include:
- Transition metal mediated carbon-carbon bond formation reactions
- Use of stabilized carbanions, ylides and enamines for carbon-carbon bond formation reactions,
- Advanced level use of oxidation and reduction reagents in synthesis.
As a modern text, this book stands out from its competitors due to its comprehensive coverage of recently published research. The book contains specific examples from the latest literature, covering modern reactions and the latest procedural modifications. The focus on contemporary and synthetically useful reactions ensures that the contents are specifically relevant and attractive to postgraduate students and industrial organic chemists.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Chapter 1
Synthetic Strategies
1.1 An introduction to organic synthesis
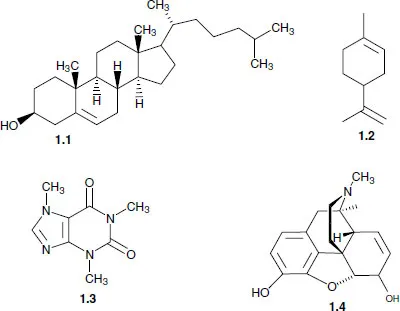
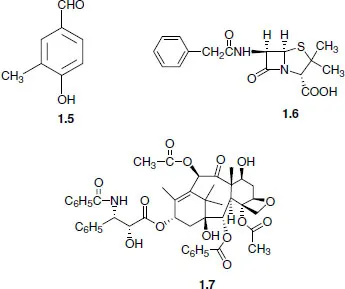
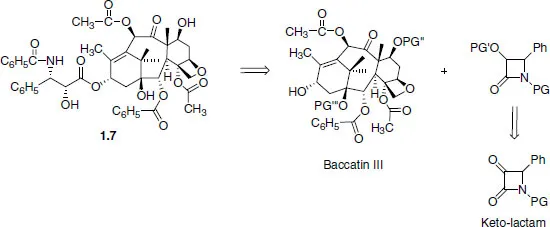
1.2 Retrosynthetic analysis (disconnection approach)
| Direction | Synthetic | Retrosynthetic |
| Step | Reaction | Transform or retro-reaction |
| Arrow used in graphical depiction | → | ⇒ |
| Starting structure | Reactant | Target |
| Resulting structure | Product | Precursor |
| Substructure required for operation | Reacting functionality | Retron |
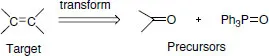
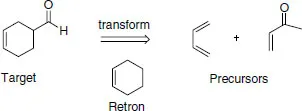
Table of contents
- Cover
- Contents
- Series
- Title
- Copyright
- Dedication
- Foreword
- Preface
- About the Author
- Abbreviations
- Chapter 1: Synthetic Strategies
- Chapter 2: Reactive Intermediates
- Chapter 3: Stabilized Carbanions, Enamines and Ylides
- Chapter 4: Carbon–Carbon Double Bond Forming Reactions
- Chapter 5: Transition Metal-Mediated Carbon–Carbon Bond Forming Reactions
- Chapter 6: Reduction
- Chapter 7: Oxidation
- Chapter 8: Pericyclic Reactions
- Index