
eBook - ePub
The Chemistry of Heterocycles
Structures, Reactions, Synthesis, and Applications
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
The Chemistry of Heterocycles
Structures, Reactions, Synthesis, and Applications
About this book
This classical textbook in the best sense of the word is now completely revised, updated and with more than 40% new content. The approved
ordering system according to the ring size of the heterocycles has been retained, while the important chapter on 'Problems and their Solutions' has been almost completely renewed by introduction of up-to-date scientific exercises, resulting in a great tool for self-testing and exams. There was maintained a chapter on nomenclature and a helpful index of name reactions. With approximately 1,000 new literature citations, this book remains a brilliant gateway to modern heterocyclic science for master and graduate students, as well as PhDs and researchers entering the field.
'If you want quick information about the basic (or acidic!) properties of a heterocycle, some interesting facts, or an assorted few ways of making
it, this book provides a welcoming, accurate, and concise introduction.'
Angewandte Chemie IE
'Eicher and Hauptmann provide an up to date introduction to the field for the advanced undergraduate and graduate students. ... The book is carefully produced to a very high standard.'
European Journal of Medicinal Chemistry
ordering system according to the ring size of the heterocycles has been retained, while the important chapter on 'Problems and their Solutions' has been almost completely renewed by introduction of up-to-date scientific exercises, resulting in a great tool for self-testing and exams. There was maintained a chapter on nomenclature and a helpful index of name reactions. With approximately 1,000 new literature citations, this book remains a brilliant gateway to modern heterocyclic science for master and graduate students, as well as PhDs and researchers entering the field.
'If you want quick information about the basic (or acidic!) properties of a heterocycle, some interesting facts, or an assorted few ways of making
it, this book provides a welcoming, accurate, and concise introduction.'
Angewandte Chemie IE
'Eicher and Hauptmann provide an up to date introduction to the field for the advanced undergraduate and graduate students. ... The book is carefully produced to a very high standard.'
European Journal of Medicinal Chemistry
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access The Chemistry of Heterocycles by Theophil Eicher,Siegfried Hauptmann,Andreas Speicher in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Physical & Theoretical Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
The Structure of Heterocyclic Compounds
Most chemical compounds consist of molecules. The classification of such chemical compounds is based on the structure of these molecules, which is defined by the type and number of atoms as well as by the covalent bonding within them. There are two main types of structure:
- The atoms form a chain–aliphatic (acyclic) compounds
- The atoms form a ring–cyclic compounds.
Cyclic compounds in which the ring is made up of atoms of one element only are called isocyclic compounds, for example, 1. If the ring consists of C-atoms only, then we speak of a carbocyclic compound, for example, 2. Cyclic compounds with at least two different atoms in the ring (as ring atoms or members of the ring) are known as heterocyclic compounds. The ring itself is called a heterocycle. If the ring contains no C-atom, then we speak of an inorganic heterocycle, for example, 3. If at least one ring atom is a C-atom, then the molecule is an organic heterocyclic compound for example, 4. In this case, all the ring atoms which are not carbon are called heteroatoms.

Along with the type of ring atoms, their total number is important, since this determines the ring size. The smallest possible ring is three-membered. The most important rings are the five- and six-membered heterocycles. There is no upper limit; there exist seven-, eight-, nine-, and larger-membered heterocycles.
In principle, all elements except the alkali metals can act as ring atoms. Although inorganic heterocycles have been synthesized, this book limits itself to organic ones. In these, the N-atom is the most common heteroatom. Next in importance are O- and S-atoms. Heterocycles with Se-, Te-, P-, As-, Sb-, Bi-, Si-, Ge-, Sn-, Pb-, or B-atoms are less common.
To determine the stability and reactivity of heterocyclic compounds, it is useful to compare them with their carbocyclic analogs. In principle, it is possible to derive every heterocycle from a carbocyclic compound by replacing appropriate CH2 or CH groups by heteroatoms. If one limits oneself to monocyclic systems, one can distinguish four types of heterocycles as follows:
1 Saturated Heterocycles (Heterocycloalkanes), e.g.

In this category, there are no multiple bonds between the ring atoms. The compounds react largely like their aliphatic analogs, for example, oxane (tetrahydropyran) and dioxane behave like dialkyl ethers, thiane and 1,4-dithiane like dialkyl sulfides, and piperidine and piperazine like secondary aliphatic amines.
2 Partially Unsaturated Systems (Heterocycloalkenes), e.g.

If the multiple bonds are between two C-atoms of the ring, as, for instance, in 3,4-dihydro-2H-pyran (6), the compounds react essentially like alkenes or alkynes, enolethers, enamines, and so on. The heteroatom can also be part of a double bond. In the case of X = O+, the compounds behave like oxenium salts, in the case of X = S+ like sulfenium salts, and in the case of X = N like imines (azomethines).
3 Systems with the Greatest Possible Number of Noncumulated Double Bonds (Heteroannulenes), e.g.
From the annulenes, one can formally derive two types of heterocycles:
- systems of the same ring size, if CH is replaced by X
- systems of the next lower ring size, if HC=CH is replaced by X.

In both cases, the resulting heterocycles are iso-π-electronic with the corresponding annulenes, that is, the number of π-electrons in the ring is the same. This is because in the pyrylium and thiinium salts, as well as in pyridine, pyrimidine, azocine, and 1,3-diazocine, each heteroatom donates one electron to the conjugated system and its nonbonding electron pair does not contribute. However, with furan, thiophene, pyrrole, oxepin, thiepin, and azepine, one electron pair of the heteroatom is incorporated into the conjugated system (delocalization of the electrons). Where nitrogen is the heteroatom, this difference can be expressed by the designation pyridine-like N-atom or pyrrole-like N-atom. In imidazole both types can be found.
4 Heteroaromatic Systems
This includes heteroannulenes, which comply with the HÜCKEL rule, that is, which possess (4n + 2) π-electrons delocalized over the ring. The most important group of these compounds derives from [6]annulene (benzene). They are known as heteroarenes, for example, furan, thiophene, pyrrole, pyridine, and the pyrylium and thiinium ions. As regards stability and reactivity, they can be compared to the corresponding benzenoid compounds [1a–d].
The antiaromatic systems, that is, systems possessing 4n delocalized electrons, for example, oxepine, azepine, thiepine, azocine, and 1,3-diazocine, as well as the corresponding annulenes, are, by contrast, much less stable and very reactive.
The classification of heterocycles as heterocycloalkanes, heterocycloalkenes, heteroannulenes, and heteroaromatics allows an estimation of their stability and reactivity. In some cases, this can also be applied to inorganic heterocycles. For instance, borazine (3), a colorless liquid, bp 55 °C, is classified as a heteroaromatic system.
Reference
1 (a) von Rague Schleyer, P. and Jiao, H. (1996) Pure Appl. Chem., 68, 209; (b) von Rague Schleyer, P. and Jiao, H. (2001) Chem. Rev., 101, 1115; (c) Bird, C.W. (1998) Tetrahedron, 54, 10179; (d) Krygowski, T.M., Cyranski, M.K., Czarnocki, Z., Häfelinger, G., and Katritzky, A.R. (2000) Tetrahedron, 56, 1783.
Chapter 2
Systematic Nomenclature of Heterocyclic Compounds
Many organic compounds, including heterocyclic compounds, have a trivial name. This usually originates from the compounds occurrence, its first preparation, or its special properties.
| Structure | Trivial name | Systematic name (IUPAC) |
 | ethylene oxide | oxirane |
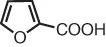 | pyromucic acid | furan-2-carboxylic acid |
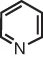 | pyridine | pyridine (instead of azine) |
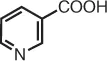 | nicotinic acid | pyridine-3-carboxylic acid |
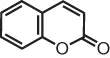 | coumarin | 2H-chromen-2-one |
The derivation of the systematic name of a heterocyclic compound is based on its structure. Nomenclature rules have been drawn up by the IUPAC Commission and these should be applied when writing theses, dissertations, publications, and patents. These rules are listed in Section R-2 of the IUPAC “Blue Book” together with worked examples (H. R. Panico, W. H. Powell, J.-C. Richer, A Guide to IUPAC Nomenclature of Organic Compounds, Recommendations 1993; Blackwell Scientific: Oxford, 1993; the previous IUPAC Blue Book: J. Rigandy, S. P. Klesney Nomenclature of Organic Chemistry; Pergamon: Oxford, 1979).
The IUPAC rules are not given in detail here, rather instructions are given for formulating systematic names with appropriate reference to the Blue Book.
Every heterocyclic compound can be refer...
Table of contents
- Cover
- Related Titles
- Title Page
- Copyright
- Preface to the Third Edition
- Abbreviations and Symbols
- Chapter 1: The Structure of Heterocyclic Compounds
- Chapter 2: Systematic Nomenclature of Heterocyclic Compounds
- Chapter 3: Three-Membered Heterocycles
- Chapter 4: Four-Membered Heterocyles
- Chapter 5: Five-Membered Heterocycles
- Chapter 6: Six-Membered Heterocycles
- Chapter 7: Seven-Membered Heterocycles
- Chapter 8: Larger Ring Heterocycles
- Chapter 9: Problems and Their Solutions
- Index