![]()
Chapter 1
Renal Disease
John W. Stirling1 and Alan Curry2
1Centre for Ultrastructural Pathology, IMVS—SA Pathology Adelaide Australia
2Health Protection Agency, Clinical Sciences Building Manchester Royal Infirmary, Manchester United Kingdom
1.1 The Role of Transmission Electron Microscopy (TEM) in Renal Diagnostics
The ultrastructural examination of renal biopsies has made a significant contribution to our understanding of renal disease and is fundamental to accurate diagnosis. For overall tissue evaluation, light microscopy (LM), immunolabelling and transmission electron microscopy (TEM) are generally combined as an integrated protocol. LM is used to make an assessment of overall tissue morphology and to identify the major pathological processes present. Immunolabelling (preferably using immunofluorescence or by the immunoperoxidase technique) is used to determine the composition and location of glomerular immune deposits. Local practices vary, but an antibody panel can contain antibodies directed against IgG, IgA, IgM, complement (C3, C1q and sometimes C4), κ and λ light chains and albumin. TEM can play a major role when LM and immunolabelling findings are normal, only mildly atypical or equivocal and difficult to interpret, particularly in respect to conditions where there may be similar LM or immunolabelling findings. Thus, the technique is particularly useful in the setting of familial disease where the structural abnormalities in the glomerular basement membrane (GBM) cannot be resolved by LM (e.g. Alport's syndrome). TEM can also provide critical information not revealed by the other methodologies to identify underlying primary disease and unexpected concomitant disease. Similarly with immunolabelling, the full classification and staging of deposits require ultrastructural analysis. Some transplant biopsies can also benefit from ultrastructural evaluation (see Chapter 2); however, TEM rarely contributes to the diagnosis of tubular, vascular or interstitial disease. Overall, ultrastructural screening is essential; it can change the diagnosis in ∼25% of cases and provides ‘useful’ information in ∼66% of cases (Pearson et al., 1994; Elhefnawy, 2011).
1.2 Ultrastructural Evaluation and Interpretation
Examination of glomeruli (and other areas, if necessary) should be thorough and systematic with all components being evaluated for possibly significant features or changes. During screening, a range of representative images should be taken. These should include low-power images to show overall glomerular morphology, plus a representative selection of higher power images to show the specific and critical diagnostic features. In some instances, it may also be important to show that certain features are, in fact, absent (e.g. deposits) or normal (e.g. foot processes). The principal elements that should be examined are (i) the location, size and morphology of immune-related deposits and other inclusions; (ii) the thickness, overall morphology and texture of the GBM; (iii) the size and morphology of the mesangial matrix and (iv) the number and morphology of the cellular components of the glomerulus (Stirling et al., 2000). Sclerotic glomeruli should be avoided, and only well-preserved functional (or significantly functional) glomeruli should be examined. It is also important to ensure that the glomeruli screened are representative of the LM findings: this means that, ideally, the choice of glomeruli to be screened (from semithin sections) should be done in collaboration with the reporting pathologist. Finally, it should be stressed that screening should be unbiased, although some knowledge of the pathology and immunolabelling results may be useful if the features expected are minor or uncommon. The vascular pole should be avoided during ultrastructural evaluation as it may contain misleading nonpathologic deposits, and likewise Bowman's capsule which has no real diagnostic value, although the presence of crescents can be confirmed.
Following evaluation, representative images and findings should be communicated to the reporting pathologist, the latter verbally or in a concise written report. If the initial evaluation does not correspond with the LM evaluation (e.g. the electron microscopy (EM) samples only a tiny fraction of the available tissue), then the specimen should be re-examined or additional glomeruli observed to increase diagnostic confidence.
A critical question is ‘How many glomeruli should be examined, and for how long?’ Unfortunately, there is no definitive answer to this dilemma except to say that enough tissue should be examined to answer the diagnostic question posed and to ensure that no additional or unexpected pathology is present. A single glomerulus (or even part of one) may be adequate in respect to diffuse disease and/or when the glomerulus screened is typical of the disease process identified by LM. In contrast, several glomeruli, or possibly glomeruli from different blocks, may be required to capture the full range of pathological changes in focal disease. Perhaps the final word on this issue is to say that the tissue must be screened thoroughly; it is bad practice to stop screening once the features that were expected have been located because additional findings that affect the accuracy of the diagnosis may be missed.
1.3 The Normal Glomerulus
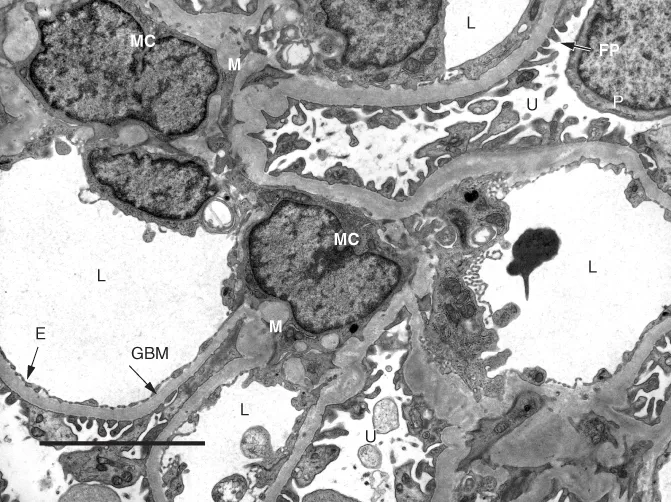
The glomerulus (Figure 1.1) is composed of a tuft of branching capillaries that originate from the afferent arteriole at the vascular pole to form a series of lobules (segments) that ultimately rejoin at the vascular pole and exit the glomerulus via the efferent arteriole. At the core of each lobule is the mesangium which supports the capillary loops; capillary loops are lined by endothelial cells (Figure 1.1). The mesangial matrix principally consists of collagen IV and is populated by mesangial cells (usually 1–3 in normal mesangium) plus a small number of immune-competent cells and rare transient cells of the monocyte–macrophage lineage (Sterzel et al., 1982). The entire capillary tuft is enclosed within Bowman's capsule, the inner aspect of which is lined by a thin layer of epithelial cells (the parietal epithelial cells); a second inner population of epithelial cells (the visceral epithelial cells or podocytes) is closely associated with the capillary tufts, and extensions of these cells form the foot processes (pedicels) that cover the outer aspect of the capillary walls (Figure 1.1). The podocytes are the sole source of the collagen IV α3, α4 and α5 subtypes that form the bulk of the GBM (Abrahamson et al., 2009), and the foot processes play a major role in ultrafiltration and the maintenance of the filtration barrier. As a result, podocyte dysfunction plays a major role in a wide range of glomerular diseases (Wiggins, 2007; Haraldsson, Nystrom and Deen, 2008). Opposite the vascular pole, Bowman's capsule is continuous with the proximal tubule which drains filtrate from the glomerulus (the urinary pole). Overall, filtration is said to be a function of size, shape and charge selection, although the nature and contribution of charge selection are debated (Harvey et al., 2007; Haraldsson, Nystrom and Deen, 2008; Goldberg et al., 2009). The capillary wall as a whole is responsible for the filtration process, and it appears that the capillary endothelium, the GBM and the podocyte foot processes must all be intact for normal filtration to occur (Patrakka and Tryggvason, 2010).
1.3.1 The Glomerular Basement Membrane
The GBM (Figure 1.1) is made of three layers: (i) the lamina rara interna, the electron-lucent layer immediately adjacent to the endothelium; (ii) the lamina densa, the central layer and (iii) the lamina rara externa, the outer electron-lucent area immediately adjacent to the foot processes. The lamina densa makes up the bulk of the GBM and is its main structural element; it has a felt-like fibrillar construction, and knowledge of its molecular makeup is helpful in understanding and interpreting familial and autoimmune disease. The principal component is collagen IV, which consists of six subtypes (α1–α6) (Patrakka and Tryggvason, 2010). In the developing kidney, the GBM is initially formed of the α1 and α2 subtypes with the α3, α4 and α5 subtypes forming later (the additional subtype, α6, is restricted to Bowman's capsule and some tubular basement membranes) (Harvey et al., 1998; Miner, 1998). In the mature kidney, the α1 and α2 subtypes are restricted to a narrow band immediately adjacent to the capillary endothelium; the α3, α4 and α5 subtypes form the remaining bulk of the GBM (extending out to the foot processes). The core of the mesangial matrix is composed of the α1 and α2 subtypes (continuous with the inner aspect of the GBM), while the outer peripheral layer is made up of the α3, α4 and α5 subtypes (continuous with the outer layer of the GBM) (Butkowski et al., 1989; Harvey et al., 1998; Miner, 1998). The α3, α4 and α5 subtypes are essential for the maintenance of normal glomerular function, and mutations in the genes for these subtypes are responsible for the various forms of membrane-related hereditary nephritis. The structural abnormalities of the GBM in hereditary disease are caused by the absence of the α3 and α5 subtypes, because without either of these, the membrane fails to form correctly (Kalluri et al., 1997; LeBleu et al., 2010; Miller et al., 2010). The α3 subtype has been identified as the Goodpasture epitope (Saus et al., 1988). However, it appears that both the α3 and α5 subtypes are targeted in anti-GBM disease, while in Alport's post-transplantation...