
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Chemical Synthesis of Nucleoside Analogues
About this book
Compiles current tested and proven approaches to synthesize novel nucleoside analogues
Featuring contributions from leading synthetic chemists from around the world, this book brings together and describes tested and proven approaches for the chemical synthesis of common families of nucleoside analogues. Readers will learn to create new nucleoside analogues with desired therapeutic properties by using a variety of methods to chemically modify natural nucleosides, including:
- Changes to the heterocyclic base
- Modification of substituents at the sugar ring
- Replacement of the furanose ring by a different carbo- or heterocyclic ring
- Introduction of conformational restrictions
- Synthesis of enantiomers
- Preparation of hydrolitically stable C-nucleosides
Chemical Synthesis of Nucleoside Analogues covers all the major classes of nucleosides, including pronucleotides, C-nucleosides, carbanucleosides, and PNA monomers which have shown great promise as starting points for the synthesis of nucleoside analogues. The book also includes experimental procedures for key reactions related to the synthesis of nucleoside analogues, providing a valuable tool for the preparation of a number of different compounds.
Throughout the book, chemical schemes and figures help readers better understand the chemical structures of nucleoside analogues and the methods used to synthesize them. Extensive references serve as a gateway to the growing body of original research studies and reviews in the field.
Synthetically modified nucleosides have proven their value as therapeutic drugs, in particular as antiviral and antitumor agents. However, many of these nucleoside analogues have undesirable side effects. With Chemical Synthesis of Nucleoside Analogues as their guide, researchers have a new tool for synthesizing a new generation of nucleoside analogues that can be used as therapeutic drugs with fewer unwanted side effects.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Chapter 1: Biocatalytic Methodologies for Selective Modified Nucleosides
1.1 Introduction
| Accepted Name (Abbreviation) |
| Other Denominations |
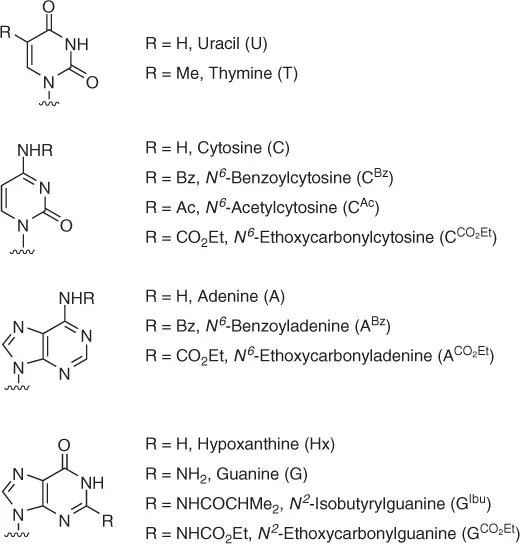
| Adenosine deaminase (ADA) Adenylate deaminase (AMPDA) 5′-adenylic acid deaminase, AMP deaminase Candida antarctica lipase A (CAL-A) lipase A Candida antarctica lipase B (CAL-B) Novozym-435, SP-435, lipase B Candida rugosa lipase (CRL) Candida cylindracea lipase (CCL) ChiroCLEC BL Lipase M (from Mucor javanicus) Lipozyme Mucor miehei lipase, Lipozyme IM Pseudomanas cepacia lipase (PSL) Pseudomonas sp. lipase, Pseudomonas fluorescens lipase (PFL), PCL, lipase P, lipase PS, LPS, amano PS, amano lipase PS Burkholderia cepacia Pig liver esterase (PLE) Porcine pancreas lipase (PPL) Subtilisin Thermomyces lanuginosa lipase (TL IM) Lipozyme TL IM |
1.2 Transformations on the sugar moiety
1.2.1 Enzymatic Acylation/Hydrolysis
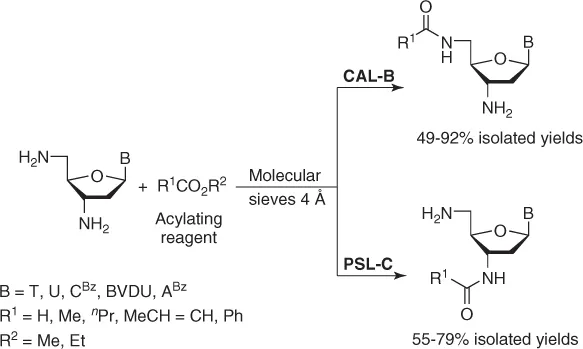
Table of contents
- Cover
- Title Page
- Copyright
- Dedication
- Contributors
- Foreword
- Preface
- Chapter 1: Biocatalytic Methodologies for Selective Modified Nucleosides
- Chapter 2: Nucleosides Modified at the Base Moiety
- Chapter 3: Chemical Synthesis of Acyclic Nucleosides
- Chapter 4: Phosphonated Nucleoside Analogues
- Chapter 5: Chemical Syntheses of Nucleoside Triphosphates
- Chapter 6: Mononucleotide Prodrug Synthetic Strategies
- Chapter 7: Synthesis of C-Nucleosides
- Chapter 8: Methodologies for the Synthesis of Isomeric Nucleosides and Nucleotides of Antiviral Significance
- Chapter 9: Synthesis of Conformationally Constrained Nucleoside Analogues
- Chapter 10: Synthesis of 3′-Spiro-Substituted Nucleosides: Chemistry of TSAO Nucleoside Derivatives
- Chapter 11: l-Nucleosides
- Chapter 12: Chemical Synthesis of Carbocyclic Analogues of Nucleosides
- Chapter 13: Uncommon Three-, Four-, and Six-Membered Nucleosides
- Chapter 14: Recent Advances in Synthesis and Biological Activity of 4′-Thionucleosides
- Chapter 15: Recent Advances in the Chemical Synthesis of Aza-Nucleosides
- Chapter 16: Stereoselective Methods in the Synthesis of Bioactive Oxathiolane and Dioxolane Nucleosides
- Chapter 17: Isoxazolidinyl Nucleosides
- Chapter 18: Synthetic Studies on Antifungal Peptidyl Nucleoside Antibiotics
- Chapter 19: Chemical Synthesis of Conformationally Constrained PNA Monomers
- Index