
Industrial Moisture and Humidity Measurement
A Practical Guide
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
Moisture analysis covers a variety of methods for measuring high levels of moisture, as well as trace amounts, in solids, liquids, or gases. There are many applications where trace moisture measurements are indispensable for manufacturing and for process quality assurance. Trace moisture in solids must be controlled for plastics, pharmaceuticals and heat treatment processes. Measurement applications in gases and liquids include, for example, drying processes, hydrocarbon processing, pure gases in the semiconductor industry, natural gas pipeline transport, the conditioning of food and other products. Written by experts with over 20 years of experience in the field, this one-stop guide covers all aspects of these measurements, including both the theory and a wealth of practical know-how. As such, it includes guidelines on installation, on the realization of standards for absolute and relative humidity, verification and traceability measurements, equipment calibration methods and the latest research developments. Backed by numerous case studies, this practical book serves the needs of those working in the industry tasked with performing or developing new techniques and processes for moisture and humidity measurement. As a result, the scientist or engineer has all the information required for accurate, reliable, economically viable and efficient moisture measurement.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1.1 Water as a Natural Resource
- for the generation of energy,
- for the transportation of people and goods,
- as a building material,
- in industrial manufacturing processes,
- for relaxing and recreation,
- for the removal of waste,
- as a cleaning agent, and
- for the interim or final storage of many different materials.
1.2 Physical and Chemical Properties of Water
1.2.1 The Water Molecule
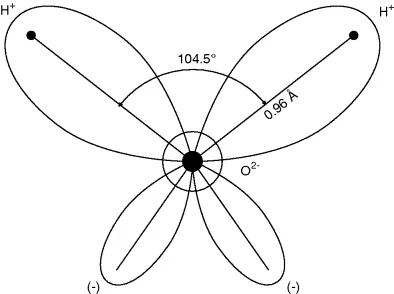
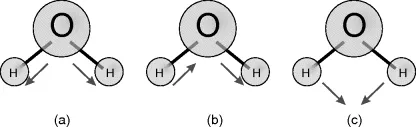
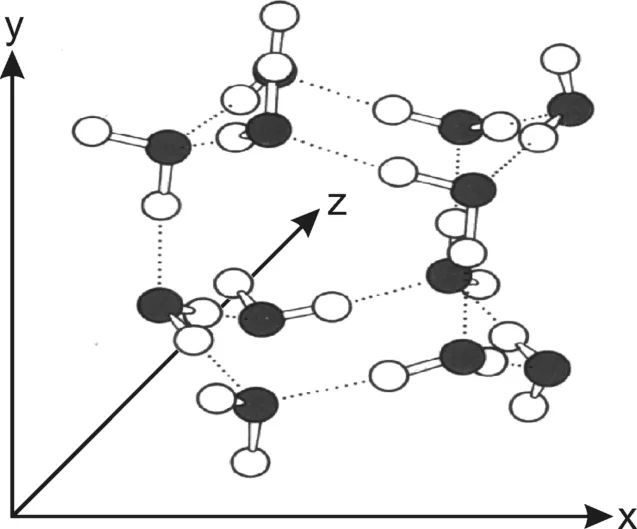
- H2: 0.23–0.29 nm,
- N2: 0.32–0.36 nm,
- O2: 0.29–0.35 nm, and
- CO2: 0.33 nm,
1.2.2 Physical Properties
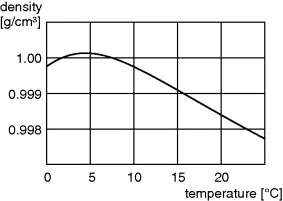
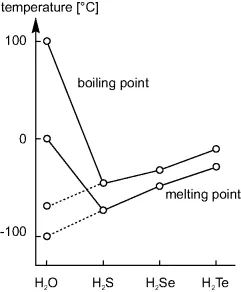
1.2.3 Chemical Properties
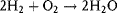
- it is soluble in many substances and forms free ions by dissociation,
- it can be easily absorbed due to the low molar mass of the molecule,
- the dipole of the molecules allows for the formation of stable bonds, and
- the hydrogen bonds cause an interlinking with other polar molecules.
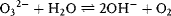
Table of contents
- Cover
- Related Titles
- Title Page
- Copyright
- Preface
- Chapter 1: Water – Substance of Life
- Chapter 2: Thermodynamic Terms and Definitions
- Chapter 3: Water in Solid, Liquid, and Gaseous Materials
- Chapter 4: Moisture and Humidity Measurement Methods in Solid, Liquid, and Gaseous Substances
- Chapter 5: Selection of a Measurement Method
- Chapter 6: Reliability and Traceability of Measurements
- Chapter 7: Moisture Measurement in Meteorology, Agriculture, and the Environment
- Chapter 8: Applications in the Food and Beverage Industry
- Chapter 9: Moisture and Humidity Measurement in Industrial Plants
- Chapter 10: Applications in the Chemical, Pharmaceutical, and Plastics Industries
- Chapter 11: Applications in the Manufacture and Processing of Paper and Textiles
- Chapter 12: Moisture Measurement in the Building Industry
- Chapter 13: Laboratory-Based Moisture Measurement
- Chapter 14: Moisture and Humidity Measurement in Space
- Appendix A: Relevant Units of Thermodynamics
- Appendix B: Tables and Diagrams of Thermodynamics
- Appendix C: Constants and Parameters
- Appendix D: Material Parameters
- Appendix E: Water Adsorption in Products
- Index