
Oxidation of Amino Acids, Peptides, and Proteins
Kinetics and Mechanism
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Oxidation of Amino Acids, Peptides, and Proteins
Kinetics and Mechanism
About this book
Explains the role of reactive intermediates in biological systems as well as in environmental remediation
With its clear and systematic approach, this book examined the broad range of reactive intermediate that can be generated in biological environments, detailing the fundamental properties of each reactive intermediate. Readers gain a contemporary understanding of how these intermediates react with different compounds, with an emphasis on amino acids, peptides, and proteins. The author not only sets forth the basic chemistry and nature of reactive intermediates, he also demonstrates how the properties of the intermediates presented in the book compare with each other.
Oxidation of Amino Acids, Peptides, and Proteins begins with a discussion of radical and non-radical reactive species as well as an exploration of the significance of reactive species in the atmosphere, disinfection processes, and environmental remediation. Next, the book covers such topics as:
- Thermodynamics of amino acids and reactive species and the effect of metal-ligand binding in oxidation chemistry
- Kinetics and mechanisms of reactive halogen, oxygen, nitrogen, carbon, sulfur and phosphate species as well as reactive high-valent Cr, Mn, and Fe species
- Reactivity of the species with molecules of biological and environmental importance
- Generation of reactive species in the laboratory for kinetics studies
- Oxidation of amino acids, peptides, and proteins by permanganate, ferryl, and ferrate species
- Application of reactive species in purifying water and treating wastewater
With this book as their guide, readers will be able to assess the overall effects of reactive intermediates in biological environments. Moreover, they'll learn how to apply this knowledge for successful water purification and wastewater treatment.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
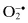
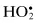
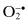
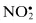
| Free Radicals | Nonradicals |
| Reactive oxygen species (ROS) | |
Superoxide, 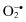 | Hydrogen peroxide, H2O2 |
| Hydroxyl, •OH | Hypobromous acid, HOBra |
Hydroperoxyl, 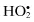 | Hypochlorous acid, HOClb |
Carbonate, 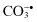 | Ozone, O3 c |
Peroxyl, 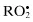 | Singlet 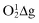 |
| Alkoxyl, RO• | Organic peroxides, ROOH |
Carbon dioxide radical, 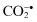 | Peroxynitrite, ONOO− d |
Singlet 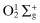 | Peroxynitrate, O2NOO− d |
| Peroxynitrous acid, ONOOH _ | |
Peroxomonocarbonate, 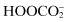 | |
| Nitrosoperoxycarbonate, ONOOCO2 | |
| Reactive nitrogen species (RNS) | |
| Nitric oxide, NO• | Nitrous acid, HNO2 |
| Nitrogen dioxide, NO2 c | Nitrosyl cation, NO+ |
Nitrate, 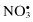 | Nitroxyl anion, NO− |
| Dinitrogen tetroxide, N2O4 | |
| Dinitrogen trioxide, N2O3 | |
| Peroxynitrite, ONOO− d | |
| Peroxynitrate, O2NOO− | |
| Peroxynitrous acid, ONOOHd | |
Nitronium cation, 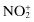 | |
| Alkyl peroxynitrites, ROONO | |
| Alkyl peroxynitrates, RO2ONO | |
| Nitryl chloride, NO2Cl | |
Peroxyacetyl nitrate, 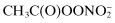 | |
| Reactive chlorine species (RCS) | |
| Atomic chlorine, Cl• | Hypochlorous acid, HOClb |
| Nitryl chloride, NO2Cle | |
| Chloramines | |
| Chlorine gas (Cl2) | |
| Bromine chloride (BrCl)a | |
| Chlorine dioxide (ClO2) | |
| Reactive bromine species (RBS) | |
| Atomic bromine, Br• | Hypobromous acid (HOBr) |
| Bromine gas (Br2) | |
| Bromine chloride (BrCl) | |
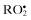
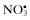
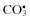
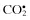
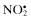
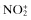
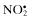
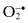
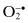
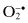
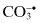
1.1 DISEASES
1.1.1 Neurodegenerative Diseases
Table of contents
- COVER
- WILEY SERIES OF REACTIVE INTERMEDIATES IN CHEMISTRY AND BIOLOGY
- TITLE PAGE
- COPYRIGHT PAGE
- DEDICATION
- PREFACE TO SERIES
- INTRODUCTION
- 1 REACTIVE SPECIES
- 2 ACID–BASE PROPERTIES
- 3 HALOGENATED SPECIES
- 4 REACTIVE OXYGEN SPECIES
- 5 REACTIVE INORGANIC OXY-SPECIES OF C, N, S, AND P
- 6 HIGH-VALENT Cr, Mn, AND Fe SPECIES
- INDEX