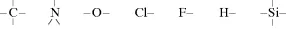![]()
Chapter 1
Introduction
Although relatively new to the scene of materials science, polymers have become ubiquitous over the past century. In fact, since the Second World War, polymeric materials represent the fastest growing segment of the U.S.' chemical industry. It has been estimated that more than a third of the chemical research dollar is spent on polymers, with a correspondingly large proportion of technical personnel working in the area. From the beginning, the study of polymers was an interdisciplinary science, with chemists, chemical engineers, mechanical engineers, and materials scientists working to understand the chemical structure and synthesis of polymers, develop methods to scale up and process polymers, and evaluate the wide range of mechanical properties existing within the realm of polymeric materials. The molecular structure of polymers is far more complex than the molecules you may have studied in a general chemistry course: just compare the molecular weights, H2O is 18, NaCl is about 58, but polymers have molecular weights from 10,000 to tens of millions (or possibly much higher for cross-linked polymers). Many of the structures you might have seen in a general cell biology course are made of polymers––proteins, polysaccharides, and DNA are all notable biological polymers. In a material science course, you may have studied crystal structures in metals to understand the mechanical behavior of different alloys (polymers can form crystals, too, but imagine the difficulty of trying to line up a huge polymer molecule into a crystal structure). Polymers are a unique class of materials having wide ranging applications.
A modern automobile contains over 300 lb (150 kg) of plastics, and this does not include paints, the rubber in tires, or the fibers in tires and upholstery. Newer aircraft incorporate increasing amounts of polymers and polymer-based composites. With the need to save fuel and therefore weight, polymers will continue to replace traditional materials in the automotive and aircraft industries. Similarly, the applications of polymers in the building construction industry (piping, resilient flooring, siding, thermal and electrical insulation, paints, decorative laminates) are already impressive and will become even more so in the future. A trip through your local supercenter will quickly convince anyone of the importance of polymers in the packaging (bottles, films, trays), clothing (even cotton is a polymer), and electronics industries. Many other examples from pharmaceutical coatings to playground equipment could be cited, but to make a long story short, the use of polymers now outstrips that of metals not just on a volume basis but also on a mass basis.
People have objected to synthetic polymers because they are not “natural.” Well, botulism is natural, but it is not particularly desirable. Seriously, if all the polyester and nylon fibers in use today were to be replaced by cotton and wool, their closest natural counterparts, calculations show that there would not be enough arable land left to feed the populace, and we would be overrun by sheep. The fact is that there simply are no practical natural substitutes for many of the synthetic polymers used in modern society.
Since most modern polymers have their origins in petroleum, it has been argued that this increased reliance on polymers constitutes an unnecessary drain on energy resources. However, the raw materials for polymers account for less than 2% of total petroleum and natural gas consumption, so even the total elimination of synthetic polymers would not contribute significantly to the conservation of hydrocarbon resources. Furthermore, when total energy costs (raw materials plus energy to manufacture and ship) are compared, the polymeric item often comes out well ahead of its traditional counterpart, for example, glass versus plastic beverage bottles. In addition, the manufacturing processes used to produce polymers often generate considerably less environmental pollution than the processes used to produce the traditional counterparts, for example, polyethylene film versus brown kraft paper for packaging.
Ironically, one of the most valuable properties of polymers, their chemical inertness, causes problems because polymers do not normally degrade in the environment. As a result, they increasingly contribute to litter and the consumption of scarce landfill space. One of the challenges in using polymers in materials is developing suitable methods for recycle or effective methods to improve the degradation of disposable items.
Environmentally degradable polymers are being developed, although this is basically a wasteful approach and we are not yet sure of the impact of the degradation products. Burning polymer waste for its fuel value makes more sense, because the polymers retain essentially the same heating value as the raw hydrocarbons from which they were made. Still, the polymers must be collected and this approach wastes the value added in manufacturing the polymers.
This ultimate solution is recycling. If waste polymers are to be recycled, they must first be collected. Unfortunately, there are literally dozens (maybe hundreds) of different polymers in the waste mix, and mixed polymers have mechanical properties similar to Cheddar cheese. Thus, for anything but the least- demanding applications (e.g., parking bumpers, flower pots), the waste mix must be separated prior to recycling. To this end, several automobile manufacturers have standardized plastics used in cars that can be easily removed, remolded, and reused in newer models. Another identifier helpful in recycling plastics is obvious if you have ever looked at the bottom of a plastic soda bottle; there are molded-in numbers on most of the large volume commodity plastics, allowing hand sorting of different materials.
Processes have been developed to separate the mixed plastics in the waste. The simplest of these is a sink–float scheme that takes advantage of density differences among various plastics. Unfortunately, many plastic items are foamed, plated, or filled (mixed with nonpolymer components), which complicates density-based separations. Other separation processes are based on solubility differences between various polymers. An intermediate approach chemically degrades the waste polymer to the starting materials from which new polymer can be made. Other efforts related to polymeric waste have focused on reducing the seemingly infinite lifetime of many plastics in the environment by developing biodegradable commodity polymers.
There are five major areas of application for polymers: (1) plastics, (2) rubbers or elastomers, (3) fibers, (4) surface finishes and protective coatings, and (5) adhesives. Despite the fact that all five applications are based on polymers, and in many cases the same polymer is used in two or more, the industries pretty much grew up separately. It was only after Dr. Harmann Staudinger [1,2] proposed the “macromolecular hypothesis” in the 1920s explaining the common molecular makeup of these materials (for which he won the 1953 Nobel Prize in chemistry in belated recognition of the importance of his work) that polymer science began to evolve from the independent technologies. Thus, a sound fundamental basis was established for continued technological advances. The history of polymer science is treated in detail elsewhere [3,4].
Economic considerations alone would be sufficient to justify the impressive scientific and technological efforts expended on polymers in the past several decades. In addition, however, this class of materials possesses many interesting and useful properties completely different from those of the more traditional engineering materials and that cannot be explained or handled in design situations by the traditional approaches. A description of three simple experiments should make this obvious.
1. Silly putty, a silicone polymer, bounces like rubber when rolled into a ball and dropped. On the other hand, if the ball is placed on a table, it will gradually spread to a puddle. The material behaves as an elastic solid under certain conditions and as a viscous liquid under others.
2. If a weight is suspended from a rubber band, and the band is then heated (taking care not to burn it), the rubber band will contract appreciably. All materials other than polymers will undergo thermal expansion upon heating (assuming that no phase transformation has occurred over the temperature range).
3. When a rotating rod is immersed in a molten polymer or a fairly concentrated polymer solution, the liquid will actually climb up the rod. This phenomenon, the Weissenberg effect, is contrary to what is observed in nonpolymer liquids, which develop a curved surface profile with a lowest point at the rod, as the material is flung outward by centrifugal force.
Although such behavior is unusual in terms of the more familiar materials, it is a perfectly logical consequence of the molecular structure of polymers. This molecular structure is the key to an understanding of the science and technology of polymers and will underlie the chapters to follow.
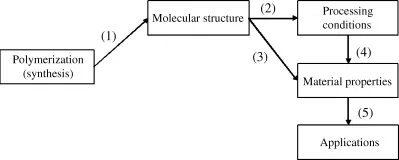
Figure 1.1 illustrates the followings questions to be considered:
1. How is the desired molecular structure obtained?
2. How do the polymer's processing (i.e., formability) properties depend on its molecular structure?
3. How do its material properties (mechanical, chemical, optical, etc.) depend on molecular structure?
4. How do material properties depend on a polymer's processing history?
5. How do its applications depend on its material properties?
The word polymer comes from the Greek word meaning “many- membered.” Strictly speaking, it could be applied to any large molecule formed from a relatively large number of smaller units or “mers,” for example, a sodium chloride crystal, but it is most commonly (and exclusively, here) restricted to materials in which the mers are held together by covalent bonding, that is, shared electrons. For our purposes, only a few bond valences need be remembered:
It is always a good idea to “count the bonds” in any structure written to make sure they conform to the above.
Example 1.1 Carbon is the most common element in polymers. Why?
Solutio...