
Nanomaterials in Drug Delivery, Imaging, and Tissue Engineering
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Nanomaterials in Drug Delivery, Imaging, and Tissue Engineering
About this book
This comprehensive volume provides the reader valuable insight into the major areas of biomedical nanomaterials, advanced nanomedicine, nanotheragnostics, and cutting-edge nanoscaffolds.
The ability to control the structure of materials allows scientists to accomplish what once appeared impossible before the advent of nanotechnology. It is now possible to generate nanoscopic self-assembled and self-destructive robots for effective utilization in therapeutics, diagnostics, and biomedical implants. Nanoscopic therapeutic systems incorporate therapeutic agents, molecular targeting, and diagnostic imaging capabilities and they have emerged as the next generation of multifarious nanomedicine to improve the therapeutic outcome including chemo and translational therapy.
Nanomaterials in Drug Delivery, Imaging, and Tissue Engineering comprises fifteen chapters authored by senior scientists, and is one of the first books to cover nanotheragnostics, which is the new developmental edge of nanomedicine combining both diagnostic and therapeutic elements at the nano level. This large multidisciplinary reference work has four main parts: biomedical nanomaterials; advanced nanomedicine; nanotheragnostics; and nanoscaffolds technology.
This groundbreaking volume also covers:
- Multifunctional polymeric nanostructures for therapy and diagnosis
- Metalla-assemblies acting as drug carriers
- Nanomaterials for management of lung disorders and drug delivery
- Responsive polymer-inorganic hybrid nanogels for optical sensing, imaging, and drug delivery
- Core/shell nanoparticles for drug delivery and diagnosis
- Theranostic nanoparticles for cancer imaging and therapy
- Magnetic nanoparticles in tissue regeneration
- Core-sheath fibers for regenerative medicine
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Part I
BIOMEDICAL NANOMATERIALS
Chapter 1
Nanoemulsions: Preparation, Stability and Application in Biosciences
Abstract
1.1 Introduction
- Classical nanoemulsions were the first lipid nanospheres to be introduced decades ago. These systems were composed of a liquid lipid core, stabilized by a membrane of surfactants. Despite the interest of these initially developed systems for solubilization of lipophilic actives in aqueous phases, few finally reached the market due to formulation issues. Indeed, these systems used to suffer from low colloidal stability (even though this has been dramatically improved as will be shown here), and sustained release of encapsulated actives is difficult to achieve due to the low viscosity of the dispersed phase [12], high surface/volume ratio, and low rigidity of the surfactants membrane. This generally leads to the rapid diffusion of the drugs out of the droplets.
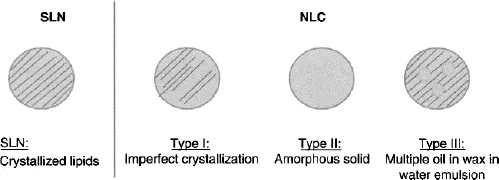
- Solid Lipid Nanoparticles (SLN) have thus been proposed to overcome these limitations. These systems present a structure identical to nanoemulsions. However, the internal lipids forming the nanoparticle core are crystalline lipids here, conferring a solid nature to the particle’s core (Figure 1.1). SLN are thus generally composed of pure long chain triglycerides, wax or long chain carboxylic acids [8]. They can be stabilized by all types of surfactants; their choice being principally dictated by the administration route envisaged [8]. SLN fabrication processes are similar to nanoemulsions, but the lipid phase is generally heated above the lipids fusion temperature to favor droplet size reduction. Lipid crystallization then occurs following cooling and storage. However, despite high expectations of such systems for prolonged release of hydrophobic molecules, SLN have shown limited controllability. As will be further explained, crystallization of the lipid phase generally leads to active/lipid phase separation and subsequent expulsion, providing high burst release [13, 14].
- Nanostructured Lipid Carriers (NLC) were introduced as a compromise. Composed of a mixture of liquid and solid lipids, the NLC core presents an imperfect crystallization which favors better encapsulation ratio thanks to lower crystallinity, while allowing control over release kinetics through the solid character of the lipid phase [15]. Three different types of NLC have been proposed (Figure 1.1): 1) the imperfect type, whose crystallinity is lowered by creating imperfections in the crystal lattices; 2) the structureless type, which is solid but amorphous; and 3) the multiple O/F/W type, in which small droplets of liquid lipids are phase separated in the solid matrix [16]. Although these 3 types can theoretically be obtained, the mixture of spatially incompatible lipids generally leads to the obtainment of the first type NLC [17, 18]. Several studies have reported the obtainment of the forms II and III, however, there is discrepancy in the conclusions. Some authors thus account for supercooled melt rather than amorphous solid particles concerning the structureless type [15, 19, 20]. Similarly, the same system was described as a typical multiple oil in fat in water type (O/F/W) [21], while a complete demixing of oil from wax occurs in the so-called “nanospoon structure” [22–24].
1.2 Nanoemulsion: A Thermodynamic Definition and Its Practical Implications
1.2.1 Generalities on Emulsions
- Interest: Emulsions are largely used to solubilize and transport substances in a continuous phase in which they are normally not soluble: hydrophobic substances can, for instance, be easily solubilized in water without the use of any solvents [25, 26]. Such an approach is of high interest for surface treatment like route surfacing or painting; the aim being to depose hydrophobic substances onto surfaces through a continuous water phase that will evaporate, and form a film by fusion between adjacent droplets. Emulsions are also widely used for their rheological properties: it is indeed possible to change liquid solutions into semi-solid formulations such as gels or creams, property largely used in the food and pharmaceutical/cosmetics industry; or, inversely, to make macroscopic solid become easily spreadable, such as bitumen for route surfacing.
- Production: Most emulsion systems generally require energy for their formation. One part of it allows overcoming the surface-free energy required to increase the interface between the two phases (ΔG = γΔA; with ΔG, free energy of the system; γ, surface tension; ΔA, created interface area) and finely disperse one phase into the other. The other important part is also used to overcome the viscous resistance along the scission of large globules into small droplets. Finally, the last part is simply lost through dissipation by the Joule effect. Different methods exist and are already used at a laboratory- and industrial-scale: mechanical agitation, high pressure homogenizer or ultrasonication and microfluidics systems [25, 26].
- Stability: Once formed, emulsions can present highly different life-time, depending on their composition and their production procedure. This stability can thus vary from a few hours to more than a year [25, 27, 28]. Among the most important parameters are the mutual solubility of the two phases and the surfactant(s) type(s) and concentration(s) [25, 27]. Indeed, different phenomena can lead the system to destabilize: some of them rely on particles aggregation and gravitation, and are reversible; others, related to droplets size evolutio...
Table of contents
- Cover
- Half Title page
- Title page
- Copyright page
- Preface
- List of Contributors
- Part I: Biomedical Nanomaterials
- Part II: Advanced Nanomedicine
- Part III: Nanotheragnostics
- Part IV: Nanoscaffolds Technology
- Index