
Handbook of Engineering and Specialty Thermoplastics, Volume 1
Polyolefins and Styrenics
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Handbook of Engineering and Specialty Thermoplastics, Volume 1
Polyolefins and Styrenics
About this book
This book focuses on common types of polymers belonging to the class of polyolefins and styrenics. The text is arranged according to the chemical constitution of polymers and reviews the developments that have taken place in the last decade. A brief introduction to the polymer type is given and previous monographs and reviews dealing with the topic are listed for quick reference. The text continues with monomers, polymerization, fabrication techniques, properties, application, as well as safety issues.
Providing a rather encyclopedic approach to polyolefins and styrenics, The Handbook of Engineering and Specialty Thermoplastics:
-
Presents a listing of suppliers and commercial grades
-
Reviews current patent literature, essential for the engineer developing new products
-
Contains as extensive tradenames index with information that is fairly unique
-
Concludes with an index of acronyms
The Handbook of Engineering and Specialty Thermoplastics: Polyolefins and Styrenics provides a comprehensive reference for chemical engineers and offers advanced students with a textbook for use in courses on chemically biased plastics technology and polymer science.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1
Metathesis Polymers
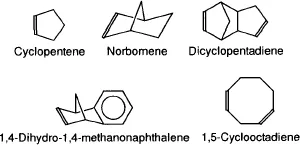
| Monomers | References |
| Cyclopentene | |
| 1,5-Cyclooctadiene | |
| Norbornene | (1,2) |
| 1,4-Dihydro-1,4-methanonaphthalene | |
| Norbornene 2-ethylhexyl carboxylate | (5) |
| Norbornene isobornyl carboxylate | (5) |
| Norbornene phenoxyethyl carboxylate | (5) |
| Dodecylenedinorbornene dicarboxyimide | (5) |
| exo, exo-N,N′-Propylene-di-(norbomene-5,6-dicarboxyimide | (5) |
| 8-Methyltetracyclo[4.4.0.12.8.17.10]dodeca-3-ene | (6) |
| Dicyclopentadiene | (6) |
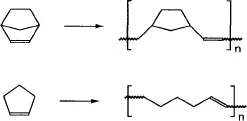
1.1 Monomers
1.2 Polymerization and Fabrication
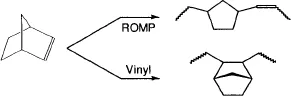
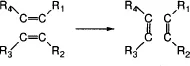
1.2.1 Metathesis Reaction
| Term | Acronym |
| Ring opening metatheses polymerization | ROMP |
| Living ring opening metatheses polymerization | LROMP (11,12) |
| Ring closing metathesis | RCM |
| Acyclic diene metathesis polymerization | ADMET |
| Ring opening metathesis | ROM |
| Cross-metathesis | CM or XMET |
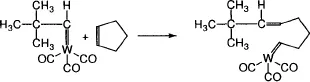
- Monomer-polymer equilibrium, in more general sense,
- Equilibrium between polymers of different chain length,
- Ring-chain equilibrium, and
- Cis-trans-equilibrium.
| Monomer! | Ratea | b |
| Cyclopentenec | 1,590 | 2.05d |
| Bicyclo[2.2.1]heptene-2c | 1,365 | 1.88d |
| 5-Cyano-5-methyl-bicyclo[2.2.1]heptene-2 | 1,365 | 1.22e |
| 3,6-Methylene-1,2,3,6-tetrahydro-cis-phthalic anhydride | 1,283 | 0.97e |
| 2,3-Diethoxycarbonyl-bicyclo[2.2.1]hepta-2,5-diene | 1,264 | 1.17e |
| 1,5-Cyclooctadienec | 1,202 | 1.98d |
| N-Phenyl-3,6-methylene-1,2,3,6-tetrahydro-cis-phthalimide | 1,182 | 1.05d |
| N-Butyl-3,6-methylene-1,2,3,6-tetrahydro-cis-phthalimide | 1,121 | 1.07e |
| 5,6-Dimethoxycarbonyl-bicyclo[2.2.1]heptene-2 | 1,039 | 0.70e |
| 5-(4-Quinolyl)-bicyclo[2.2.1]heptene-2 | 998 | 0.81e |
| 5-Acetoxy-bicyclo[2.2.1]heptene-2 | 978 | 0.85e |
| 5-Methoxymethylbicyclo[2.2.1]heptene-2 | 978 | 0.69e |
| N,N-Diethyl-bicyclo[2.2.1]heptene-2-carbonamide | 937 | 0.94e |
| 1,4-Dihydro-1,4-methanonaphthalene | 897 | 0.78d |
| 5-Chloromethyl-bicyclo[2.2.1]heptene-2 | 876 | 0.80d |
| 5-(2-Pyridyl)-bicyclo[2.2.1]heptene-2 | 876 | 0.81e |
| 5,5-Dichloro-bicyclo[2.2.1]heptene-2 | 815 | 1.11d |
Table of contents
- Cover
- Title Page
- Copyright
- Preface
- Contents
- Chapter 1 - Metathesis Polymers
- Chapter 2 - Cyclic Olefin Copolymers
- Chapter 3 - Ultra High Molecular Weight Poly(ethylene)
- Chapter 4 - Poly(methyl)pentene
- Chapter 5 - Ionomers
- Chapter 6 - Poly(isobutylene)
- Chapter 7 - Ethylene Vinyl Acetate Copolymers
- Chapter 8 - Acrylonitrile-Butadiene-Styrene Polymers
- Chapter 9 - High Impact Poly(styrene)
- Chapter 10 - Styrene/Acrylonitrile Polymers
- Chapter 11 - Methyl methacrylate/Butadiene/Styrene Polymers
- Chapter 12 - Acrylonitrile/Styrene/Acrylate Polymers
- Index