
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Handbook of Heterogenous Kinetics
About this book
This book presents all the theoretical and practical basements of heterogeneous kinetics and reactivity of solids. It applies the new concepts of reactivity and spatial function, introduced by the author, for both nucleation and growth processes, with aunified presentation of the reactivity of bulk and powder solids, including gas-solid reactions, thermal decompositions, solid-solid reactions, reactions of solid solutions, and coalescence of solid grains. It also containsmany exercises and problems with solutions included, allowing readers to understand and use all the concepts and methods discussed therein.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Handbook of Heterogenous Kinetics by Michel Soustelle in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Technology & Engineering Research & Skills. We have over one million books available in our catalogue for you to explore.
Information
Edition
1Chapter 1
Definitions and Experimental Approach
This chapter describes the kinetics of heterogenous systems containing solids. We initially give a classification of these systems and recall some basic notions. And above all, we present an overview of experimental facts that modeling enables us to rediscover.
1.1. Thermal transformations of solids
The subject of our study is to understand the development of phenomena involving the heating of solids in a specific environment.
Transforming a solid means modifying one or more of its features. A transformation can therefore be defined by the description of:
- the initial state: the chemical species, their phases, their amount and aspect (massive or in powder form); and
- the end state: chemical species and their phases.
For instance, heating calcium carbonate is not transformation, but the reaction CaCO3 = CaO + CO2 in which a known mass of calcite powder produces lime powder is transformation.
The increase in size of anatase grains is a grain-growth transformation.
The dehydration of pentahydrated copper sulfate leads to various products, depending on the reaction conditions. This reaction is a transformation only if the final product is known (anhydrous copper sulfate, other hydrated copper sulfates).
A transformation occurs only in a space of given intensive parameters, such as temperature, partial pressures, concentrations, and total pressure, as allowed by thermodynamics (see Chapter 3).
DEFINITION.- A wholly identified transformation taking place following a given mechanism (see section 7.2.2) is called a “process”.
Therefore, two processes occur during the above-mentioned decarbonizing of calcium carbonate: nucleation and growth. Anatase grain growth may happen through different mechanisms, such as volume diffusion, in bulk or through the surface, or through gaseous phase, and so on.
The kinetic study of a thermal transformation has to take place under conditions allowed by thermodynamics and needs a precise definition of the system. The aim of the study is to determine the processes involved in the transformation and their mechanisms. This study leads to a speed equation expressed as a function of various variables, including time.
This study needs the characterization of initial and final products and of intermediate states. These characterizations must be chemical (composition), structural (nature and composition of the phases), and textural (solid area, porosity, and shape and size of the solid). The chemical surface of the solid is also to be characterized (surface acidity, adsorbed species, etc.).
REMARK.- A component of a chemical system is an identified chemical species in a given phase.
1.2. Classification of transformations
When considering the mechanism, it is useful to classify the thermal transformation of solids into families, including classes and subclasses. Each family, class, or subclass of transformation will present common points even if each transformation has its own characteristics. Therefore, similar ways of study and phenomena description can be applied for each family of transformation.
Two main families appear first: transformations without formation of a new solid phase (such as anatase grain growth mentioned earlier) and transformations that lead to a new solid phase (such as decarbonizing described previously).
The first family is divided into two classes: transformations that modify phase composition and transformations with only textural change.
The second family is also divided into two classes. In the first one, the initial solid is the single reactant. In the second one, the transformation involves several reactants. Some subclasses will precisely describe these two classes.
1.2.1. Transformation without formation of a new solid phase
In this transformation, the initial solid phase surrounded by a gaseous phase is preserved, but the rise in temperature induces a change in the composition of the solid, or a textural modification due to grain growth (which can lead to densification and sintering).
1.2.1.1. Solid phase composition change
Stoichiometric change due to reaction of gas with a solid belongs to this class of transformation, such as the stoichiometric variation in cerium oxide under oxygen pressure, which can be expressed as
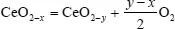
Note that this form (see section 2.5.1) is not to be used.
This class of transformation also includes the change in the composition of a solid solution either by release of a gas or by reaction of one of the components, leading to a gaseous compound. Gas adsorption and gas desorption also belong to this class of transformation.
1.2.1.2. Solid phase textural change
Heating fine grains of powdered solids leads to their coalescence (similar to what happens when two mercury drops come in contact), which is called “grain growth.” A chemical equation cannot describe this phenomenon, but it is in fact a transformation. This phenomenon is harmful for the catalytic converter because it leads to a large decrease in the catalyst support area and therefore a drop in the catalytic converter’s efficacy.
Another textural transformation is sintering of, or densification by heating of, a powder, which leads to the formation of a massive solid from a powdered one. This transformation is often used for manufacturing ceramic pieces.
1.2.2. Transformation with formation of a new solid phase
This family includes all the transformations starting with a solid A as reactant and producing another solid B on the surface of solid A.
We can divide such transformations into two classes, depending on whether solid A is a single reactant or whether it reacts with other species belonging to other phases.
1.2.2.1. The initial solid is a single reactant
This class includes three subclasses that are described hereafter.
1.2.2.1.1. Polymorphic transformation
In this transformation, solid A is transformed into solid B having the same composition but another structure, that is, another crystal lattice. Let us take as examples the transformation of α sulfur into β sulfur and the transformation of titanium dioxide with anatase structure into titanium dioxide with rutile structure according to
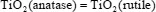
1.2.2.1.2. Thermal decomposition
In this transformation, heating of solid A produces a new solid B having a distinct composition and structure. As in the case of calcium carbonate mentioned earlier, this transformation leads to release of one or several gases.
1.2.2.1.3. Precipitations of new phases
As the initial phase contains several components, heating (or cooling) induces the precipitation of a new solid phase from the components of the initial phase, which therefore have a new composition. (This transformation is sometimes called “decomposition”.) As an example, we can mention the decomposition of solid solutions of cerium and zirconium dioxides, with a high content of ceria and therefore having the structure of ceria, into monoclinic zirconia according to
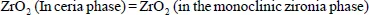
Small amounts of gas may be released during such decomposition. It is therefore similar to the precipitation of a solid phase from a solution made up of a salt and water with the removal of the latter in gaseous form (crystallization by evaporation).
1.2.2.2. The initial sol...
Table of contents
- Cover
- Title Page
- Copyright
- Preface
- List of Symbols
- Chapter 1: Definitions and Experimental Approach
- Chapter 2: The Real Solid: Structure Elements and Quasi-Chemical Reactions
- Chapter 3: Thermodynamics of Heterogenous Systems
- Chapter 4: Elementary Steps in Heterogenous Reactions
- Chapter 5: Chemical Diffusion
- Chapter 6: Chemical Adsorption
- Chapter 7: Mechanisms and Kinetics of a Process
- Chapter 8: Nucleation of a New Solid Phase
- Chapter 9: Growth of a Solid Phase
- Chapter 10: Transformation by Surface Nucleation and Growth
- Chapter 11: Modeling and Experiments
- Chapter 12: Granular Coalescence
- Chapter 13: Decomposition Reactions of Solids
- Chapter 14: Reactions Between Solids
- Chapter 15: Gas-Solid Reactions
- Chapter 16: Transformations of Solid Solutions
- Chapter 17: Modeling of Mechanisms
- Chapter 18: Mechanisms and Kinetic Laws
- Chapter 19: Mechanisms and Reactivity
- Appendix 1: Sample Shapes
- Appendix 2: Space Functions of Anisotropic Growths for a Grain
- Appendix 3: Laws of Evolutions in the One-Process Models with Instantaneous Nucleation and Anisotropic Growth
- Appendix 4: Kinetic Laws in Two-Process Models with Anisotropic Growth
- Appendix 5: Tables of Values in Two-Process Models with Anisotropic Growth
- Appendix 6: Tables of Values in Some Two-Process Models with Isotropic Growth
- Appendix 7: Tables of Values in Some Two-Process Models with Isotropic Growth
- Appendix 8: Kinetics and Mechanisms with Parallel Steps
- Appendix 9: Reaction with Nucleation in the Bulk
- Appendix 10: Mathematical Complements
- Appendix 11: Physical Units and Constants
- Bibliography
- Index