
eBook - ePub
Molecular Fluorescence
Principles and Applications
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Molecular Fluorescence
This second edition of the well-established bestseller is completely updated and revised with approximately 30 % additional material, including two new chapters on applications, which has seen the most significant developments.
The comprehensive overview written at an introductory level covers fundamental aspects, principles of instrumentation and practical applications, while providing many valuable tips.
For photochemists and photophysicists, physical chemists, molecular physicists, biophysicists, biochemists and biologists, lecturers and students of chemistry, physics, and biology.
Information
1
Introduction
| … ex arte calcinati, et illuminato aeri seu solis radiis, seu flammae fulgoribus expositi, lucem inde sine calore concipiunt in sese; … | [… properly calcinated, and illuminated either by sunlight or flames, they conceive light from themselves without heat; …] |
Licetus, 1640 (about the Bologna stone)
1.1 What Is Luminescence?
The word luminescence, which comes from the Latin (lumen = light) was first introduced as luminescenz by the physicist and science historian Eilhardt Wiedemann in 1888, to describe “all those phenomena of light which are not solely conditioned by the rise in temperature,” as opposed to incandescence. Luminescence is often considered as cold light whereas incandescence is hot light.
Luminescence is more precisely defined as follows: spontaneous emission of radiation from an electronically excited species or from a vibrationally excited species not in thermal equilibrium with its environment.1) The various types of luminescence are classified according to the mode of excitation (see Table 1.1).
Table 1.1 The various types of luminescence.
| Phenomenon | Mode of excitation |
| Photoluminescence (fluorescence, phosphorescence, delayed fluorescence) | Absorption of light (photons) |
| Radioluminescence | Ionizing radiation (X-rays, α, β, γ) |
| Cathodoluminescence | Cathode rays (electron beams) |
| Electroluminescence | Electric field |
| Thermoluminescence | Heating after prior storage of energy (e.g., radioactive irradiation) |
| Chemiluminescence | Chemical reaction (e.g., oxidation) |
| Bioluminescence | In vivo biochemical reaction |
| Triboluminescence | Frictional and electrostatic forces |
| Sonoluminescence | Ultrasound |
Luminescent compounds can be of very different kinds:
- Organic compounds: aromatic hydrocarbons (naphthalene, anthracene, phenanthrene, pyrene, perylene, porphyrins, phtalocyanins, etc.) and derivatives, dyes (fluorescein, rhodamines, coumarins, oxazines), polyenes, diphenylpolyenes, some amino acids (tryptophan, tyrosine, phenylalanine), etc.
- Inorganic compounds: uranyl ion (), lanthanide ions (e.g., Eu3+, Tb3+), doped glasses (e.g., with Nd, Mn, Ce, Sn, Cu, Ag), crystals (ZnS, CdS, ZnSe, CdSe, GaS, GaP, Al2O3/Cr3+ (ruby)), semiconductor nanocrystals (e.g., CdSe), metal clusters, carbon nanotubes and some fullerenes, etc.
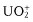
- Organometallic compounds: porphyrin metal complexes, ruthenium complexes (e.g., ), copper complexes, complexes with lanthanide ions, complexes with fluorogenic chelating agents (e.g., 8-hydroxy-quinoline, also called oxine), etc.
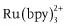
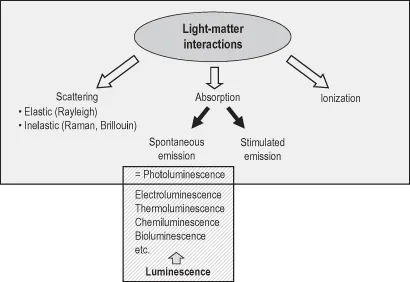
Fluorescence and phosphorescence are particular cases of luminescence (Table 1.1). The mode of excitation is absorption of one or more photons, which brings the absorbing species into an electronic excited state. The spontaneous emission of photons accompanying de-excitation is then called photoluminescence which is one of the possible physical effects resulting from interaction of light with matter, as shown in Figure 1.1. Stimulated emission of photons can also occur under certain conditions (see Chapter 3, Box 3.2). Additional processes, not shown, can take place for extremely high intensities of radiation, but are not relevant for luminescence studies.
Figure 1.1 Position of photoluminescence in the frame of light–matter interactions.
1.2 A Brief History of Fluorescence and Phosphorescence
It is worth giving a brief account of the history of fluorescence and phosphorescence. The major events from the early stages to the middle of the twentieth century are reported in Table 1.2 together with the names of the associated scientists. The story of fluorescence started with a report by N. Monardes in 1565, but scientists focused their attention on light emission phenomena other than incandescence only in the nineteenth century. However, the major experimental and theoretical aspects of fluorescence and phosphorescence were really understood only after the emergence of quantum theory, already in the twentieth century (1918–1935, i.e., less than 20 years). As in many other areas of theoretical physics and chemistry, this was an exceptionally fecund period.
Table 1.2 Milestones in the history of fluorescence and phosphorescencea).
| Year | Scientist | Observation or achievement |
| 1565 | N. Monardes | Emission of light by an infusion of the wood later called Lignum nephriticum (first report on the observation of fluorescence) |
| 1602 | V. Cascariolo | Emission of light by Bolognese stone (first detailed observation of phosphorescence) |
| 1640 | Licetus | Study of Bolognian stone. First definition as a nonthermal light emission |
| 1833 | D. Brewster | Emission of light by chlorophyll solutions and fluorspar crystals |
| 1842 | J. Herschel | Emission of light by quinine sulfate solutions (epipolic dispersion) |
| 1845 | E. Becquerel | Emission of light by calcium sulfide upon excitation in the UV |
| First statement that the emitted light is of longer wavelength than the incident light. | ||
| 1852 | G. G. Stokes | Emission of light by quinine sulfate solutions upon excitation in the UV (refrangibility of light) |
| 1853 | G. G. Stokes | Introduction of the term fluorescence |
| 1858 | E. Becquerel | First phosphoroscope. First lifetime measurements. |
| 1867 | F. Goppelsröder | First fluorometric analysis (determination of Al(III) by the fluorescence of its morin chelate) |
| 1871 | A. Von Baeyer | Synthesis of fluorescein |
| 1888 | E. Wiedemann | Introduction of the term luminescence |
| 1905, 1910 | E. L. Nichols and E. Merrit | First fluorescence excitation spectrum of a dye |
| 1907 | E.L. Nichols and E. Merrit | Mirror symmetry between absorption and fluorescence spectra |
| 1919 | O. Stern and M. Volmer | Relation for fluorescence quenching |
| 1920 | F. Weigert | Discovery of the polarization of the fluorescence emitted by dye solutions |
| 1922 | S. I. Vavilov | Excitation-wavelength independence of the fluorescence quantum yield |
| 1923 | S. I. Vavilov and W. L. Levshin | First study of the fluorescence polarization of dye solutions |
| 1924 | S. I. Vavilov | First determination of fluorescence yield of dye solutions |
| 1924 | F. Perrin | Quantitative description of static quenching (active sphere model |
| 1924 | F. Perrin | First observation of alpha phosphorescence (E-type delayed fluorescence) |
| 1925 | F. Perrin | Theory of fluorescence polarization (influence of viscosity) |
| 1925 | W. L. Levshin | Theory of polarized fluorescence and phosphorescence |
| 1925 | J. Perrin | Introduction of the term delayed fluorescence |
| Prediction of long-range energy transfer | ||
| 1926 | E. Gaviola | First direct measurement of nanosecond lifetimes by phase fluorometry (instrument built in Pringsheim’s laboratory) |
| 1926 | F. Perrin | Theory of fluorescence polarization (sphere) |
| Perrin’s equation | ||
| Indirect determination of lifetimes in solution. | ||
| Comparison with radiative lifetimes | ||
| 1927 | E. Gaviola and P. Pringsheim | Demonstration of resonance energy transfer in solutions... |
Table of contents
- Cover
- Further Titles of Interest
- Title page
- Copyright page
- Preface to the First Edition
- Preface to the Second Edition
- Acknowledgments
- Prologue
- 1 Introduction
- Part I: Principles
- Part II: Techniques
- Part III: Applications
- Appendix: Characteristics of Fluorescent Organic Compounds
- Epilogue
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Molecular Fluorescence by Bernard Valeur,Mário Nuno Berberan-Santos in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Industrial & Technical Chemistry. We have over one million books available in our catalogue for you to explore.