eBook - ePub
Statistical Physics
About this book
The Manchester Physics Series General Editors: D. J. Sandiford; F. Mandl; A. C. Phillips Department of Physics and Astronomy, University of Manchester Properties of Matter B. H. Flowers and E. Mendoza Optics Second Edition F. G. Smith and J. H. Thomson Statistical Physics Second Edition E. Mandl Electromagnetism Second Edition I. S. Grant and W. R. Phillips Statistics R. J. Barlow Solid State Physics Second Edition J. R. Hook and H. E. Hall Quantum Mechanics F. Mandl Particle Physics Second Edition B. R. Martin and G. Shaw The Physics of Stars Second Edition A. C. Phillips Computing for Scientists R. J. Barlow and A. R. Barnett
Statistical Physics, Second Edition develops a unified treatment of statistical mechanics and thermodynamics, which emphasises the statistical nature of the laws of thermodynamics and the atomic nature of matter. Prominence is given to the Gibbs distribution, leading to a simple treatment of quantum statistics and of chemical reactions. Undergraduate students of physics and related sciences will find this a stimulating account of the basic physics and its applications. Only an elementary knowledge of kinetic theory and atomic physics, as well as the rudiments of quantum theory, are presupposed for an understanding of this book. Statistical Physics, Second Edition features:
Statistical Physics, Second Edition develops a unified treatment of statistical mechanics and thermodynamics, which emphasises the statistical nature of the laws of thermodynamics and the atomic nature of matter. Prominence is given to the Gibbs distribution, leading to a simple treatment of quantum statistics and of chemical reactions. Undergraduate students of physics and related sciences will find this a stimulating account of the basic physics and its applications. Only an elementary knowledge of kinetic theory and atomic physics, as well as the rudiments of quantum theory, are presupposed for an understanding of this book. Statistical Physics, Second Edition features:
- A fully integrated treatment of thermodynamics and statistical mechanics.
- A flow diagram allowing topics to be studied in different orders or omitted altogether.
- Optional "starred" and highlighted sections containing more advanced and specialised material for the more ambitious reader.
- Sets of problems at the end of each chapter to help student understanding. Hints for solving the problems are given in an Appendix.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
CHAPTER 1
The first law of thermodynamics
1.1 MACROSCOPIC PHYSICS
Statistical physics is devoted to the study of the physical properties of macroscopic systems, i.e. systems consisting of a very large number of atoms or molecules. A piece of copper weighing a few grams or a litre of air at atmospheric pressure and room temperature are examples of macroscopic systems. In general the number of particles in such a system will be of the order of magnitude of Avogadro’s number N0 = 6 × 1023. Even if one knows the law of interaction between the particles, the enormousness of Avogadro’s number precludes handling a macroscopic system in the way in which one would treat a simple system — say planetary motion according to classical mechanics or the hydrogen molecule according to quantum mechanics. One can never obtain experimentally a complete microscopic* specification of such a system, i.e. a knowledge of some 1023 coordinates. Even if one were given this initial information, one would not be able to solve the equations of motion; some 1023 of them!
In spite of the enormous complexity of macroscopic bodies when viewed from an atomistic viewpoint, one knows from everyday experience as well as from precision experiments that macroscopic bodies obey quite definite laws. Thus when a hot and a cold body are put into thermal contact temperature equalization occurs; water at standard atmospheric pressure always boils at the same temperature (by definition called 100 °C); the pressure exerted by a dilute gas on a containing wall is given by the ideal gas laws. These examples illustrate that the laws of macroscopic bodies are quite different from those of mechanics or electromagnetic theory. They do not afford a complete microscopic description of a system (e.g. the position of each molecule of a gas at each instant of time). They provide certain macroscopic observable quantities, such as pressure or temperature. These represent averages over microscopic properties. Thus the macroscopic laws are of a statistical nature. But because of the enormous number of particles involved, the fluctuations which are an essential feature of a statistical theory turn out to be extremely small. In practice they can only be observed under very special conditions. In general they will be utterly negligible, and the statistical laws will in practice lead to statements of complete certainty.
Fig. 1.1. Gas exerting pressure on movable piston, balanced by external applied force F.
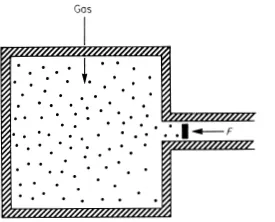
To illustrate these ideas consider the pressure exerted by a gas on the walls of a containing vessel. We measure the pressure by means of a gauge attached to the vessel. We can think of this gauge as a freely movable piston to which a variable force F is applied, for example by means of a spring (Fig. 1.1). When the piston is at rest in equilibrium the force F balances the pressure P of the gas: P = F/A where A is the area of the piston.
In contrast to this macroscopic determination of pressure consider how the pressure actually comes about.* According to the kinetic theory the molecules of the gas are undergoing elastic collisions with the walls. The pressure due to these collisions is certainly not a strictly constant time-independent quantity. On the contrary the instantaneous force acting on the piston is a rapidly fluctuating quantity. By the pressure of the gas we mean the average of this fluctuating force over a time interval sufficiently long for many collisions to have occurred in this time. We may then use the steady-state velocity distribution of the molecules to calculate the momentum transfer per unit area per unit time from the molecules to the wall, i.e. the pressure. The applied force F acting on the piston can of course only approximately balance these irregular impulses due to molecular collisions. On average the piston is at rest but it will perform small irregular vibrations about its equilibrium position as a consequence of the individual molecular collisions. These small irregular movements are known as Brownian motion (Flowers and Mendoza,26 section 4.4.2). In the case of our piston, and generally, these minute movements are totally unobservable. It is only with very small macroscopic bodies (such as tiny particles suspended in a liquid) or very sensitive apparatus (such as the very delicate suspension of a galvanometer — see section 7.9.1) that Brownian motion can be observed. It represents one of the ultimate limitations on the accuracy of measurements that can be achieved.
There are two approaches to the study of macroscopic physics. Historically the oldest approach, developed mainly in the first half of the 19th century by such men as Carnot, Clausius, William Thomson (the later Lord Kelvin), Robert Mayer and Joule, is that of classical thermodynamics. This is based on a small number of basic principles—the laws of thermodynamics—which are deductions from and generalizations of a large body of experiments on macroscopic systems. They are phenomenological laws, justified by their success in describing macroscopic phenomena. They are not derived from a microscopic picture but avoid all atomic concepts and operate exclusively with macroscopic variables, such as pressure, volume, temperature, describing the properties of systems in terms of these. Of course, the avoidance of atomic concepts severely limits the information that thermodynamics can provide about a system. In particular, the equation of state (e.g. for an ideal gas: PV=RT) which relates the macroscopic variables and which distinguishes one system from another must be derived from experiment. But there are many situations where a microscopic description is not necessary or not practicable and where thermodynamics proves its power to make far-reaching deductions of great generality.*
The second approach to macroscopic physics is that of statistical mechanics. This starts from the atomic constitution of matter and endeavours to derive the laws of macroscopic bodies from the atomic properties. This line of approach originated in Maxwell’s kinetic theory of gases which led to the profound works of Boltzmann and of Gibbs. There are two aspects to statistical mechanics. One aim is to derive the thermodynamic laws of macroscopic bodies from the laws governing their atomic behaviour. This is a fascinating but very difficult field. Nowadays one has a fairly general understanding of the underlying physics but most physicists working in the field would probably agree that no real proofs exist. In this book we shall not consider these aspects of statistical mechanics and shall only give arguments which make the thermodynamic laws plausible from the microscopic viewpoint.
The second objective of statistical mechanics is to derive the properties of a macroscopic system — for example, its equation of state — from its microscopic properties. Essentially this is done by averaging over unobservable microscopic coordinates leaving only macroscopic coordinates such as the volume of a body, as well as other macroscopic variables, such as temperature or specific heat, which have no counterpart in mechanics and which represent averages ove...
Table of contents
- Cover
- Contents
- Title Page
- Copyright
- THE MANCHESTER PHYSICS SERIES
- Editors’ preface to the Manchester Physics Series
- Preface to the Second Edition
- Preface to First Edition
- Flow diagram
- CHAPTER 1 THE FIRST LAW OF THERMODYNAMICS
- CHAPTER 2 THE SECOND LAW OF THERMODYNAMICS I
- CHAPTER 3 PARAMAGNETISM
- CHAPTER 4 THE SECOND LAW OF THERMODYNAMICS II
- CHAPTER 5 SIMPLE THERMODYNAMIC SYSTEMS
- CHAPTER 6 THE HEAT CAPACITY OF SOLIDS
- CHAPTER 7 THE PERFECT CLASSICAL GAS
- CHAPTER 8 PHASE EQUILIBRIA
- CHAPTER 9 THE PERFECT QUANTAL GAS
- CHAPTER 10 BLACK-BODY RADIATION
- CHAPTER 11 SYSTEMS WITH VARIABLE PARTICLE NUMBERS
- A MATHEMATICAL RESULTS
- B THE DENSITY OF STATES
- APPENDIX C Magnetic systems*
- APPENDIX D Hints for solving problems
- Bibliography
- Index
- Physical constants and conversion factors
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Statistical Physics by Franz Mandl in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Quantum Theory. We have over one million books available in our catalogue for you to explore.
