![]()
PART A
GENERAL CONCEPTS
![]()
CHAPTER 1
INTRODUCTION
GENERAL DISCUSSION
Materials such as plastics, glasses, and metals, are widely used in medical constructs, for example, containers, packaging systems, sets, transfer and transport systems, manufacturing systems–facilities, and devices. The physiochemical nature of these materials provides medical products with their necessary and desirable performance characteristics. A number of medical products involve constructs (objects constructed in whole or in part from materials) whose primary purpose is the generation, production, transport, storage, and/or delivery of therapeutic products that are used either directly or indirectly by patients to produce a desirable therapeutic outcome. Additionally, such constructs may be used for the same purposes with precursors of the therapeutic product. Less frequently, such constructs themselves may provide the therapeutic benefit.
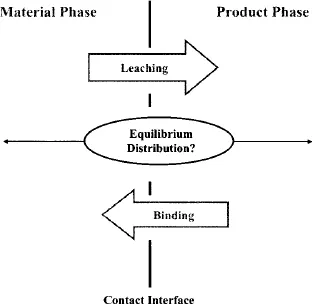
While an important performance characteristic of materials (or systems) used in medical applications is chemical inertness, interactions between a material (or system) and the pharmaceutical product it contacts are well documented. Such interactions may include sorption (binding), the uptake of product components by the material, or leaching the release of material-related components to the product (Fig. 1.1). Instances in which such an interaction can impact the therapeutic product, from either an efficacy and/or safety perspective, also have been reported. As a recent example, the leaching of a vulcanizing agent from uncoated stoppers used in prefilled syringes has been proposed as a mechanism contributing to adverse clinical events associated with EPREX®.1 Other recent examples of leachables exerting an undesirable influence on therapeutic products have also been documented.2,3 These recent examples augment a long history of instances where the safety or efficacy of a therapeutic product has been compromised by its interaction with a construct.
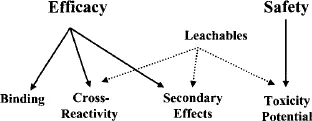
As outlined in relevant regulatory policies, procedures, and guidelines, any contact between a construct and therapeutic substance, which may or may not be a finished drug product, is an opportunity for that substance to be changed as a result of that contact. The purpose of a construct’s compatibility evaluation is to assess the magnitude, if any, of such a change. By convention, if little or no change occurs, then it is concluded that the construct and the therapeutic substance are compatible. A complete compatibility assessment considers numerous potential outcomes of the construct–substance interaction, as illustrated in Fig. 1.2. In the most general sense, specific aspects of a compatibility assessment address either the issues of a therapeutic substance’s efficacy (does the substance perform in a manner consistent with its labeling and indication) or substance safety (does the substance produce an unanticipated and adverse user response). Considering efficacy, while drug binding (loss of ingredient from the substance due to the ingredient’s uptake by a plastic construct) is the most typically documented efficacy-impacting interaction, other types of interactions are possible and significant. For example, cross-reactivity refers to the situation in which a specific entity, leached from the construct, and a substance’s ingredient interact chemically, resulting in the ingredient’s decomposition and/or inactivation. This interaction may be direct or indirect, for example, via a catalytic action. Additionally, note that efficacy does not solely reflect a substance’s ability to deliver its specified therapeutic dose. Secondary effects reflect those instances where a property of the leached entity itself has an impact on the chemical or physical characteristics of the therapeutic substance. Examples of such secondary effects include (1) an acidic or basic leachable whose accumulation pushes a substance outside of its pH specification; (2) a leachable that either directly or indirectly causes the formation of particulate matter, and (3) a leachable whose accumulation has an adverse esthetic effect [e.g., discoloration, high ultraviolet (UV) absorbance]. In the extreme situation, the secondary effect may not be manifested in an undesirable therapeutic substance, for example, a finished drug product, but rather as an undesirable construct, which may lose its ability to perform its desired function due to the construct–substance interaction.
Also note that the accumulation of leached substances in a therapeutic substance can have ramifications outside of the context of the substance’s performance. For example, leached entities can complicate substance analysis by producing analytical responses (e.g., chromatographic peaks) that either directly interfere with targeted analyte quantitation or indirectly complicate the interpretation of the analytical information.
While a complete compatibility assessment includes a consideration of therapeutic substance aspects other than safety, such considerations are beyond the scope of this book and are not considered in great detail herein (however, see Chapter 12 for a brief discussion of suitability for use aspects other than safety). Rather, the remainder of this book deals with the questions of (1) How does the leaching of substances from constructs impact the safety of a therapeutic substance, for example, a finished drug product and, more specifically; (2) How does one ascertain the magnitude of the impact? Compatibility assessments that deal with these questions are called safety assessments.
KEY DEFINITIONS
The Interacting Parties
The number of different pharmaceutical circumstances in which two entities come in contact, one of which is either directly or indirectly used to produce a favorable therapeutic outcome and the other of which is used to facilitate the generation, transport, or storage of the first, is enormous. The scale and diversity of the pharmaceutical universe creates difficulties in terms of establishing terms that can be used to generically describe interactions that arise when two entities contact one another. For example, a well-recognized entity-to-entity couple in the pharmaceutical universe is a drug product and its associated packaging system. Clearly, a drug product and its packaging system can interact; however, it is not accurate to state that all pharmaceutically relevant interactions only occur between drug products and their packaging system. What about drug products that are administered via tubing sets? What about solutions, which may be either the drug product itself or an associated precursor, whose processing includes filtration? What about contact between a production batch and its associated manufacturing apparatus (e.g., tanks or single use systems)? What about drugs products that can be solid, liquid, or gas? What about packaging systems that may consist of plastics, glasses, metals, or combinations thereof?
There is considerable value in developing a nomenclature that deals with the general case, as opposed to individual specific cases. Such a vernacular is based on the observation that any contact minimally involves two potentially interacting parties. In the pharmaceutical universe, one of the interacting parties is utilized to produce a favorable therapeutic outcome. The second interacting party is used, in one manner or another, to facilitate the generation or utilization of the party that provides the therapeutic benefit. Generic definitions for these parties are as follows:
Therapeutic Substance: A material (solid, liquid, or gas) that is used to produce a therapeutic benefit. A primary therapeutic substance is one whose use directly produces the therapeutic benefit. A secondary therapeutic substance serves as a precursor to the primary therapeutic substance. A secondary therapeutic substance is a substance that is either used and discarded to make a primary therapeutic substance and/or is further processed to produce the primary therapeutic substance. Thus, for example, a finished biopharmaceutical drug product would be a primary therapeutic substance while the growth medium in which the biopharmaceutical agent is generated is a secondary therapeutic substance.
Construct: An entity that is contacted by a therapeutic substance at some time during that substance’s lifecycle, which may include the substance’s synthesis, formulation, production, storage, or delivery. The contact between a construct and a therapeutic substance is typically associated with the product’s generation, storage, transport, or use.
While such a terminology offers the advantage of universal application, it is awkward in the sense that it falls well outside of common usage. To facilitate its interpretation, examples of therapeutic substances and their associated constructs are provided in Table 1.1.
TABLE 1.1. Examples of Constructs and Therapeutic Substances
| Therapeutic Substance | Construct |
| Dosage form (solid, liquid, or gas) | Packaging system (bags, vials, syringes, bottles, or canisters) |
| Dosage form (liquid or gas) | Transfer tubing sets |
| Process reagents (growth media or buffers) | Sterilizing filters |
| Production batches | Manufacturing equipment (tanks or single-use system) |
| Process solutions (chromatographic eluents, or cleaning agents) | Manufacturing equipment (tubing or gaskets) |
| In-process intermediates | Storage–transport containers |
| Drug compound | Drug-eluting stent |
Extractables versus Leachables
It is not uncommon to encounter the terms extractables and leachables in the context of drug compatibility assessments. Both terms are used to describe substances that migrate out of a construct when the construct is contacted with an extracting medium. While exact definitions of extractables and leachables vary slightly among the various resources that provide such definitions, these definitions all establish the same fundamental difference between these two separate, but closely related, concepts. More specifically, these terms are defined as follows:
Leachables: Substances that are present in the primary therapeutic substance because of its interaction with a material or construct during its intended use (including production, storage transport, and/or delivery).
With this definition of leachables as our foundation, the definition of extractables is straightforward. Generally, any potential migrant is an extractable. More specifically, the following definition is given:
Extractables: Substances that can be extracted from a material or construct using extraction solvents and/or extraction conditions that are expected to be at least as aggressive as the conditions of contact between the material (or construct) and a primary therapeutic substance.
Table 1.2 is provided to further clarify the difference between these two related classes of entities and provides guidance in terms of properly linking the correct term with specific study parameters. Relevant study parameters (dimensions) include the test article (the object that is extracted), the contact medium, and contact conditions. For example, the test article extracted in an extractables–leachables evaluation can be a specific raw material, a component of a construct or the actual construct itself. The contact medium can either be a solvent or a primary or secondary ...