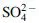![]()
Part I
Fundamentals of ES
![]()
Chapter 1
On the Mechanism of Electrospray Ionization Mass Spectrometry (ESIMS)
Paul Kebarle* and Udo H. Verkerk†
*Department of Chemistry, University of Alberta, Edmonton, Alberta, Canada
†Center for Research in Mass Spectrometry, York University, Toronto, Ontario, Canada
1.1 Introduction
1.1.1 How It All Started
1.1.2 Aims of This Chapter
1.1.3 Electrospray, Other than Mass Spectrometric Applications
1.2 Production of Gas-Phase Ions by Electrospray and Electrospray Ionization Mass Spectrometry
1.2.1 The Overall Process
1.2.2 Production of Charged Droplets at the ES Capillary Tip. The Electrophoretic Mechanism
1.2.3 Electrospray as an Electrolytic Cell of a Special Kind
1.2.4 Required Electrical Potentials for ES. Electrical Gas Discharges
1.2.5 Electrical Current, I, due to the Charged Droplets. Charge and Radius of Droplets
1.2.6 Solvent Evaporation from Charged Droplets Causes Droplet Shrinkage and Coulomb Fissions of Droplets
1.2.7 Evolution of Droplets by Evaporation and Coulomb Fissions Producing Smaller and Smaller Progeny Droplets that Lead Ultimately to Minute Charged Droplets that Produce Ions in the Gas Phase
1.2.8 Mechanisms for the Formation of Gas-Phase Ions from Very Small and Highly Charged Droplets: The Charged Residue Model (CRM) and the Ion Evaporation Model (IEM)
1.2.9 The Iribarne–Thomson Equation for Ion Evaporation from Small Charged Droplets and Subsequent Experimental and Theoretical Work Examining the Validity of IEM
1.2.10 Large Analyte Ions Such as Proteins and Dendrimers Are Most Probably Produced by the Charged Residue Model (CRM)
1.2.11 Dependence of the Observed Ion Abundance of Analytes on the Nature of the Analyte, on Its Concentration, and on the Presence of Other Electrolytes in the Solution
1.2.12 Noncovalent and Ionic Interactions in Solution and in the Gas Phase and other Relevant Differences Between Gas Phase and Solution
1.2.13 Some Examples of Effects on Mass Spectra of Proteins Due to the ESI Process
1.2.13.1 Ion Pairing of Salt ions with Ionized Residues of Proteins
1.2.13.2 Why Is Ammonium Acetate Such a Popular Salt Additive to Solutions Used for ESIMS
1.2.13.3 Determinations by Electrospray of Equilibrium Constants of Association Reactions in Solution and Possible Sources of Error Due to the ESI Process
1.2.14 Nanoelectrospray and Insights into Fundamentals of Electrospray–Nanospray
References
1.1 INTRODUCTION
1.1.1 How It All Started
Electrospray ionization (ESI) is a method by which solutes present in a solution can be transferred into the gas phase as ions. The gas-phase ions can then be detected by mass spectrometric means (ESIMS). Remarkably, ESI can handle solutes such as polymers, nucleic acids, and proteins that have a very high molecular mass such as hundreds of megadaltons for proteins. The analytes present in the solution may be ions, such as the inorganic metal ions M
+ and M
2+ protonated amines or negative ions such as the halide ions X
– or deprotonated carboxylic acids, sulfates
, and so on. They can be also compounds that are neutral in the solution that is sprayed. In that case, the analyte is charged by association with one or more ions present in the solution. This charging process is part of the electrospray mechanism. ESIMS is the ideal method for detection of analytes from high-performance liquid chromatography or capillary electrophoresis. ESIMS is particularly valuable to biochemical, biomedical, and pharmacological research. The significance of ESIMS was recognized by a Nobel Prize in 2002 to John Fenn, who was the major developer of the method.
1ESIMS is actually the brainchild of Malcolm Dole. In the 1960s, Malcolm Dole was very interested in the determination of the molecular mass of synthetic polymers and developing a method with which one could observe such macromolecules by mass spectrometry. It was clear to him that mass spectrometric analysis of the polymer molecules could answer many questions and solve many problems. But how could one get large polymers into the gas phase without decomposing them? He had the idea that if one uses a very dilute solution of the analyte and then nebulized the solution into extremely small droplets, one might obtain many droplets that contained only one analyte molecule. Evaporation of the droplets would then lead to a transfer of the analyte molecules to the gas phase. If the analyte was not charged, as was often the case for synthetic polymers, the presence of an electrolyte, such as Na+ and Cl_ in the solution, could lead to charging of the polymer. Evaporation of a droplet that happens to contain one polymer molecule and one Na+ would lead to the desired charged analyte. However, for charging to occur, there has to be one or more functional groups on the analyte with which the ion can form a fairly strongly bonded complex in the absence of the solvent. For details of such complex formation see Section 1.2.13.1. In other droplets, there would be one Cl_ and one polymer, and that would lead to a negatively charged analyte that could be observed with the mass spectrometer in the negative ion mode. Such statistical charging was known to occur2 and to be a rather inefficient source of ionized analytes.
Dole was preoccupied with thoughts on how to increase the efficiency, when a possible solution presented itself. While working as a consultant for a paint company,3 he witnessed the electrospraying of paint on automobile bodies. The paint was sprayed on the cars very efficiently by very small charged paint droplets using a process known as electrospray. Applying electrospray to polystyrene solutions, Dole et al.4 were able to develop an apparatus and demonstrate the production in the gas phase of polystyrene ions with molecular masses in the kilodalton range. While Dole’s methods and results had some flaws and ambiguities, they clearly indicated that electrospray is a very promising soft ionization method for the mass spectrometry of macromolecules.
Dole et al.’s paper4 caught the eye of Professor Seymour Lipsky at Yale Medical School. Lipsky, who was also involved with mass spectrometry, was very excited about the potential of electrospray for the mass spectrometric study of proteins. Dole et al.4 had used a nozzle-skimmer system as the interface between the atmospheric pressure required for electrospray and the mass analysis region, and their paper contained a reference to work by John Fenn, who was a specialist in the field of molecular beams and their production by nozzle-skimmer systems. Through this reference, Lipsky got in touch with Fenn, who was also at Yale but at the Department of Mechanical Engineering. This contact inspired Fenn to start research on electrospray mass spectrometry. Since only a low-mass-range quadrupole was available in Fenn’s laboratory, the first, pioneering papers5 involved studies of small ions in the positive5a and negative ion5b mode. The ES ion source and interface to the mass analysis region were similar to those used by Dole, but included a few important changes such as the use of nitrogen gas counter flow at atmospheric pressure to remove solvent vapor caused by the droplet evaporation. This measure led to clean and relatively simple mass spectra that could be easily interpreted.
Subsequent work by Fenn and co-workers,6 using a quadrupole mass analyzer with a high m/z range extending to m/z 1500 and a heated capillary as the interface between the spray at atmospheric pressure and the vacuum containing the mass analysis section, clearly demonstrated that ESIMS could be used very effectively for analysis of peptides and proteins with molecular mass m which could be much higher than m = 1500 daltons. This was possible because the use of ESI led to molecular ions that had multiple charges z so that the m/z value was lower than m/z = 1500. This work had a big impact and started the ESIMS revolution that is continuing to this day.
The development of ESIMS is clearly due to two people, Malcolm Dole and John Fenn. Dole’s early death occurred before the full impact of ESIMS became evident. Fenn,1 in his account on the development of the method, clearly acknowledges the seminal significance of Dole’s work. Much of the information in this section is based on Fenn’s account.1
1.1.2 Aims of This Chapter
This chapter is written for users of ESIMS. It presents an account of “how it all works.” It addresses those just entering the field and more advanced users. Understanding of “how it all works” is desirable not only from a standpoint of intellectual curiosity, but also for practical reasons. The mass spectra that one observes depend on a large number of parameters. These start with (a) a choice of solvent and concentrations of the analyte, (b) a choice of additives to the solution that may be beneficial, and (c) their concentration, choice of the flow rates of the solution through the spray capillary, the electrical potentials applied to the spray capillary (also called “needle”), and the potentials on the electrodes leading to the mass analysis. The choice of these parameters requires not only an understanding of conventional mass spectrometry but also an understanding of the electrospray mechanism as well as some familiarity with chemistry in solution as well as ion-molecule reactions in the gas phase. In early work on ESIMS, many of the parameters were established experimentally by trial and error; but now when a better understanding of the mechanism is at hand, it is certainly more efficient and analytically rewarding to understand the reasons for the choices.
Unfortunately, not all the processes that occur in ESIMS are well understood, and this has led to some controversy. However, enough is known to make the study of the mechanisms in ESIMS worthwhile.
The present chapter presents only a limited account of electrospray. A much more extensive coverage is provided by a review by Smith et al.7a While Smith’s review is quite old, most of the material is still very relevant.
1.1.3 Electrospray, Other than Mass Spectrometric Applications
ES existed long before its application to mass spectrometry. It is a method of considerable importance for the electrostatic dispersion of liquids and creation of aerosols. The interesting history and notable research advances in that field are very well described in Bayley’s book7b Electrostatic Spraying of Liquids. Much of the theory concerning the mechanism of the charged droplet formation was developed by researchers in aerosol science. A compilation of articles in this area can be found in a special issue7c of the Journal of Aerosol Science devoted to electrospray.
1.2 PRODUCTION OF GAS-PHASE IONS BY ELECTROSPRAY AND ELECTROSPRAY IONIZATION MASS SPECTROMETRY
1.2.1 The Overall Process
There are three major steps in the production of gas-phase ions from electrolyte ions in solution. These are: (a) production of charged droplets at the ES capillary tip; (b) shrinkage of the charged droplets by solvent evaporation and repeated droplet disintegrations leading ultimately to very small highly charged droplets capable of producing gas-phase ions; and (c) the actual mechanism by which gas-phase ions are produced from the very small and highly charged droplets. Stages (a)–(c) occur in the atmospheric pressure region of the apparatus (see Figure 1.1.).
Some of the ions resulting from the preceding stages (a)–(c) enter the vacuum region of the interface leading to the mass spectrometer either through a small orifice or through a sampling capillary (see Figure 1.2.a,b). The ions may be clustered with solvent molecules and other additives and are subjected to (i) a thermal declustering “clean-up” in the heated capillary leading to the partial vacuum (pressure of a few torr) of the first chamber and (ii) collisional activation due to an electric potential difference imposed between the sampling capillary exit and the skimmer leading to the second, high-vacuum chamber that is the housing of the mass spectrometer.