
eBook - ePub
Essential Soil Science
A Clear and Concise Introduction to Soil Science
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
This textbook is aimed at the majority of students, who need to quickly acquire a concise overview of soil science. Many current soil science textbooks still cater for a traditional student market where students embark on three years study in a narrow discipline. The growth in modular degree schemes has meant that soil science is now often taught as self-standing unit as part of broad based degree program. Students pursuing this type of course are increasingly reluctant to purchase expensive textbooks that are too detailed and often assume a scientific background. For those opting to specialise in soil science there are a variety of good textbooks to choose from. This short informative guide, will be particularly useful for students who do not possess a traditional scientific background, such as those studying geography, environment science, ecology and agriculture.
- Only textbook to cater for introductory courses in soil science.
- Provides an affordable concise overview of soil science.
- Learning exercises and chapter summaries enhance usability.
- Annotated suggestions for further reading.
- Based on proven and successful modular course structure.
- Emphasis on readability and interactive learning.
- No scientific background assumed.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Essential Soil Science by Mark Ashman,Geeta Puri in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Agronomy. We have over one million books available in our catalogue for you to explore.
Information
1
Rocks to Soil
Introduction
Let’s start with a question – what is soil and how does it form? Immediately you will know that soils are brownish, turn muddy when wet, and have some importance to plant growth. However, this level of understanding is insufficient when we really want to understand and appreciate the formation and utilization of soils for agricultural purposes. We can start by saying that rocks are transformed into soil by physical and chemical changes that occur at the Earth’s surface. Gradually, these mineral inputs are combined with organic matter. Over time both the mineral material and organic matter are transformed into new materials; these are then moved through the soil by percolating water, so that the more soluble compounds are finally lost completely. It is the nature of these inputs, transformations, movements and losses that determines what type of soil will form. This chapter will look at four main lines of enquiry:
1 Soil formation – what are the initial inputs?
What are soils made from?
What are the main mineral inputs?
Why do some rocks break up?
How do rocks break up?
What are the main organic inputs?
What are plants made from?
How does organic matter begin to accumulate in soil?
How does soil organic matter differ depending on environmental conditions?
2 How are these inputs transformed into new compounds?
What kinds of mineral material do soils contain?
What are clay minerals and how do they form?
How is organic matter transformed into new material?
What is humus and how does it form?
3 How is material moved and then finally lost from the soil?
How is soil material moved to new geographical locations?
How is material moved within the soil?
4 How can we explain soil formation?
Example one: podzol (spodosol)
Example two: gleysol (aquepts)
Example three: ferralsol (oxisol)
Example four: histosol (histosol)
1 Soil formation – what are the initial inputs?
What are soils made from?
The first step in soil formation happens when mineral material from rocks and organic matter from plants and animals are combined together. Rock fragments without organic matter are unable to support plant growth. For example, think about sowing wheat seeds in gravel – what do you think your chances of a successful crop would be? Although organic matter without mineral inputs can support plant growth, composted organic matter lacks many of the physical characteristics that are commonly associated with soil. It is the combination of mineral and organic matter that gives soil its unique properties. Together they make up approximately 50% of the soil volume: the remaining 50% is pore space, filled with either air or water depending on how wet the soil is. We will start by looking at the main mineral inputs.
What are the main mineral inputs?
Rocks are composed of one or a number of different minerals. Geologists have traditionally divided rocks into three broad classes: igneous (formed from molten magma); metamorphic (rocks that have been altered by heat and pressure); and sedimentary rocks (formed from sediment deposits). Rocks differ from each other because they contain either different types of minerals or the same minerals but in varying quantities. This concept is not difficult to understand if you compare, let’s say, natural rock and artificial building materials. We can produce a wide range of bricks, blocks and slabs, all with differing properties, by simply varying the proportions of sand, gravel and cement from which they are made: rocks are no different. Rocks with differing mineral compositions vary in their ability to withstand the natural forces that slowly disintegrate them. However, before looking at how rocks disintegrate, we should ask why they break up in the first place.
Why do some rocks break up?
The Earth’s surface is made up of many different types of rock. Some, such as carbonate rocks like limestone and chalk, are composed of prehistoric marine creatures, whereas others like coal are derived from prehistoric plants. However, in most cases rocks are mainly composed of the element silicon. In rocks, silicon is usually combined with oxygen to form silica and silicates. One important difference between rocks is the amount of silica they contain. The proportion of silica to other minerals affects rock susceptibility to disintegration when exposed at the Earth’s surface. Generally, for a given set of climatic conditions, the rocks with the lowest concentration of silica break up more quickly than those with higher concentrations. These differences in silica content occur as the molten magma solidifies to form rock. As the magma starts to cool, minerals with the lowest concentrations of silica form first. They are followed by minerals with increasing silica contents. We can use the amount of silica igneous rock contains as a useful method of classification:
- ultrabasic: rocks with less than 45% silica, such as serpentinite and peridotite;
- basic: rocks with 45–55% silica, such as basalt, gabbro and dolerite;
- intermediate: rocks with 55–65% silica, such as amphibolite and andesite;
- acidic: rocks with over 65–85% silica, such as granite and pegmatite.
How do rocks break up?
A more scientific term for the break-up of rocks is ‘weathering’. We use this term because climate, and the prevailing weather, is the main factor that eventually transforms rock to soil. Weathering can be either physical or chemical. Figure 1.1 shows how we can divide physical weathering into a number of separate processes.
Physical weathering
Thermal weathering Different minerals have different rates of expansion when heated. When rocks composed of several different minerals are exposed to heat they experience different rates of expansion. This causes stress within the rock, which can result in fracturing in areas of weakness. Thermal stress can also be caused by temperature differences between the outer and inner parts of the rock. This form of thermal stress is called exfoliation or ‘onion skin’ weathering, because as outer layers of the rock are fractured, they are gradually peeled away.
Mechanical weathering Water can penetrate rocks along small cracks. When water freezes, its volume expands by 10% (think about how uninsulated water pipes can burst in the winter). The force exerted by expanding ice is enough to break open cracks. This form of weathering is called ‘frost shattering’. In addition, when exposed to water, different minerals often have different rates of swelling and shrinkage; this can initiate stresses within the rock that eventually cause it to fracture.
Fig. 1.1 The physical break-up of rocks by thermal and mechanical means.
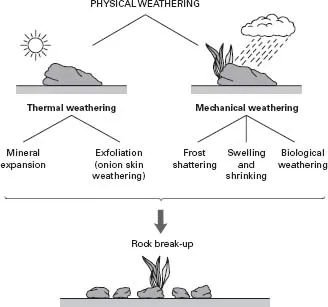
Fig. 1.2 Plants can speed up physical and chemical weathering by expanding into rock fissures and excreting substances called exudates. These are then metabolized by microorganisms, thereby increasing the rate of chemical weathering.

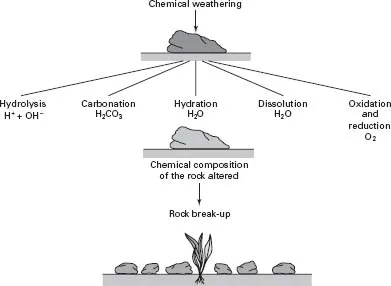
Fig. 1.3 The chemical break-up of rocks by hydrolysis, carbonation, hydration, dissolution, oxidation and reduction. Unlike physical weathering, which simply breaks the rock into smaller and smaller fragments, chemical weathering can also change the physical and chemical properties of the rock.
Another form of mechanical weathering comes from the pressure plant roots exert as they expand into crevices and small cracks in the rock. By widening existing cracks, more of the rock surface is exposed to the elements. This accelerates the weathering process and hastens the disintegration of the rock. Figure 1.2 is a good example of this process (it shows a small tree growing out of a crack in a rock).
Chemical weathering
In addition to being exposed to mechanical disruption rocks are also attacked chemically. Chemical weathering can be divided into the following set of processes (see Fig. 1.3).
Hydrolysis This is the most common form of chemical weathering. It occurs when water molecules (H2O) separate (dissociate) into two charged particles, H+ (a hydrogen ion) and OH− (a hydroxyl ion). The term ‘ion’ refers to the fact that the particle carries a charge. Hydrogen and hydroxyl ions attack the bonds that hold minerals together. Hydrolysis not only causes rock disintegration but it also changes the chemical nature of the minerals. Hydrolysis is a very important process in soils and it is essential you understand the mechanism: it is shown in Fig. 1.4.
Fig. 1.4 Hydrolysis is the chemical reaction of a compound with water. The chemical reactions for cations and anions are shown.
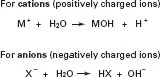
Fig. 1.5 Biological activity in the soil leads to carbon dioxide being respired. This dissolves in soil water to produce a weak acid that can then attack minerals.
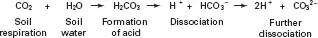
Carbonation This is an accelerated form of hydrolysis, which is caused by biological activity within the soil. The majority of soil organisms respire carbon dioxide (CO2). When CO2 comes into contact with water, a proportion of it dissolves to form carbonic acid (rain is naturally acidic for this reason). Plant roots are particularly destructive in this respect because, in addition to carbonic acid produced by respiration, they also excrete sugars that are then used and converted to acids by micro-organisms in a process not unlike tooth decay following a prolonged sugary diet. All acids are rich sources of hydrogen ions; carbonation therefore enhances hydrolysis. The process is shown in Fig. 1.5.
Hydration During hydration minerals absorb water, but unlike hydrolysis there is no ion formation: during hydration the water molecule remains intact. When a mineral undergoes hydration its physical and chemical properties can be altered. A similar process occurs when pasta is immersed in water: think about how physical characteristics are altered as it absorbs water. When some minerals become hydrated they can also become weakened physically.
Dissolution In this process minerals simply dissolve in water. A few minerals such as sodium chloride (table salt) and potassium chloride are completely soluble in water. Minerals such as these dissolve, and are then washed away in solution.
Oxidation and reduction When exposed to the atmosphere some minerals undergo chemical changes; some are ‘oxidized’ and others are ‘reduced’. In its simplest form oxidation can be regarded as a mineral’s tendency to take up oxygen, while reduction is its ability to lose oxygen. However, this narrow definition has been expanded so that it also refers to the loss (oxidation) or gain (reduction) of electrons. Although chemically the process can become quite complicated, the main point is that changes in a mineral’s oxidation state can weaken it.
Table 1.1 Broad soil carbon and nitrogen ratings.
Source: J. R. Landon (1984) Booker Tropical Soil Manual, Longman Scientific and Technical, Harlow.
| Rating | Carbon (%) | Nitrogen (%) |
| Very high | >20 | >1.0 |
| High | 10–20 | 0.5–1.0 |
| Medium | 4–10 | 0.2–0.5 |
| Low | 2–4 | 0.1–0.2 |
| Very low | <2 | <0.1 |
Although it has been convenient to subdivide weathering into chemical and physical processes, you must appreciate that they are not mutually exclusive. For example, the break-up of a large rock into smaller fragments by physical weathering also increases its rate of chemical weathering by exposing a greater proportion of its surface to the prevailing weather conditions. Put simply, in most situations smaller fragments of rock have higher rates of chemical weathering.
What are the main organic inputs?
Weathered rock finally becomes soil when organic matter is incorporated with mineral fragments. The term ‘soil organic matter’, in its widest sense, covers all the living and dead organisms contained within the soil. However, when soil scientists use the term they are usually referring to the remains of plants and animals. In some cases these residues are recent additions to the soil, while others may be many years old. When measuring soil organic matter soil scientists often refer to the concentration of soil carbon, because carbon is the main constituent of organic matter, typically accounting for around 58% of the total weight. Table 1.1 shows some typical soil carbon and nitrogen concentrations.
Soil organic matter not only supplies nutrients to plants but it also alters the physical nature of the soil by binding soil particles together into discrete units called aggregates (see Chapter 2). Before looking at soil organic matter in more detail, we will consider briefly what plant material is made from, because in most soils plant material is the commonest type of organic input.
Fig. 1.6 Plant material can be broken down into the components shown.
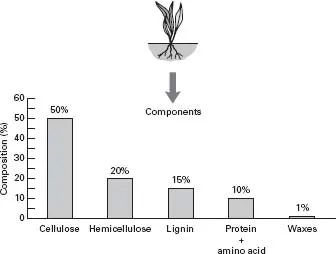
What are plants made from?
In many respects the breakdown of soil organic matter is similar to rock weathering. As with rocks, the relative proportion of resistant to degradable compounds will largely determine how quickly plant residues are broken down by soil micro-organisms. When we considered rock break-up we used the term ‘weathering’; when plant and animal residues are reduced to simple chemicals we use the term ‘mineralization’. The main compounds present in plant material are shown in Fig. 1.6.
The main compounds
Cellulose and hemicellulose Plant tissue consists mostly of cellulose (15–50%) and hemicellulose (10–30%). Plant walls are made from a combination of cellulose fibres that are encrusted with hemicelluloses. Both compounds are made up of sugar molecules joined together to form long chains.
Proteins and amino acids Proteins and amino acids also consist of carbon compounds, but unlike cellulose they also contain considerable quantities of nitrogen. Typically, plant material consists of approximately 5% protein and 5% amino acids. Nitrogen is a very important nutrient and is essential to...
Table of contents
- Cover
- Contents
- Dedication
- Title page
- Copyright
- Preface
- List of Abbrevations
- 1 Rocks to Soil
- 2 Particles, Structures and Water
- 3 Soil Surfaces, Acidity and Nutrients
- 4 Soil Microbes and Nutrient Cycling
- 5 Soil Survey, Classification and Evaluation
- 6 Soils and Agriculture
- 7 Soil Contamination and Erosion
- Further Reading
- Index