
eBook - ePub
Prodrugs and Targeted Delivery
Towards Better ADME Properties
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Prodrugs and Targeted Delivery
Towards Better ADME Properties
About this book
This topical reference and handbook addresses the chemistry, pharmacology, toxicology and the patentability of prodrugs, perfectly mirroring the integrated approach prevalent in today's drug design. It summarizes current experiences and strategies for the rational design of prodrugs, beginning at the early stages of the development process, as well as discussing organ- and site-selective prodrugs.
Every company employing medicinal chemists will be interested in this practice-oriented overview of a key strategy in modern drug discovery and development.
Every company employing medicinal chemists will be interested in this practice-oriented overview of a key strategy in modern drug discovery and development.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Prodrugs and Targeted Delivery by Jarkko Rautio, Raimund Mannhold,Hugo Kubinyi,Gerd Folkers in PDF and/or ePUB format, as well as other popular books in Medicine & Pharmacology. We have over one million books available in our catalogue for you to explore.
Information
Part One
Prodrug Design and Intellectual Property
Chapter 1
Prodrug Strategies in Drug Design
1.1 Prodrug Concept
Prodrugs are bioreversible derivatives of pharmacologically active agents that must undergo an enzymatic and/or chemical transformation in vivo to release the active parent drug, which can then elicit its desired pharmacological effect [1–4]. According to this strict definition, active agents whose metabolites contribute to a pharmacological response and salts of active drugs, which have sometimes mistakenly been referred to as prodrugs, are not considered to be prodrugs. In most cases, prodrugs are simple chemical derivatives that are one or two chemical or enzymatic steps away from the active parent drug. Some prodrugs lack an obvious carrier or promoiety, but result from a molecular modification of the active drug itself in vivo. Such a modification can be, for example, a metabolic oxidation or reduction that generates a new and active compound. These prodrugs are usually referred to as “bioprecursor prodrugs.” In some cases, a prodrug may consist of two pharmacologically active drugs that are coupled together in a single molecule, so that each drug acts as a promoiety for the other. Such derivatives are called “codrugs” [5]. Finally, “soft drugs,” which are often confused with prodrugs, also find applications in tissue targeting. In contrast to prodrugs, soft drugs are active drugs as such but are designed to transform into an inactive form in vivo after achieving their therapeutic effect [6]. The prodrug concept is illustrated in Figure 1.1.
Figure 1.1 Simplified representation of the prodrug concept. The drug–promoiety molecule is the prodrug that is typically inactive pharmacologically. In broad terms, the barrier can be thought of as any biological liability for a parent drug that prevents optimal (bio)pharmaceutical or pharmacokinetic performance. This barrier must be overcome in order to achieve a marketable drug.
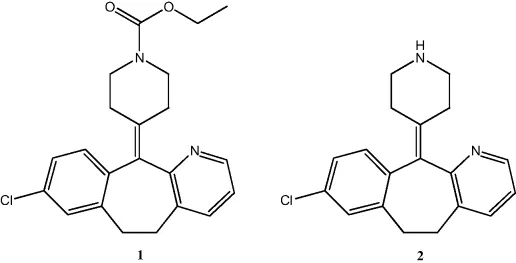
Prodrugs have been classified according to several criteria; these being, for example, based on therapeutic categories, or based on categories of chemical linkages between the parent drug and the promoiety, or based on mechanism of action of a prodrug. A recently proposed more systematic approach categorizes prodrugs on the basis of their two cellular sites of conversion: intracellular (e.g., antiviral nucleoside analogues and statins) and extracellular be it in digestive fluids or the systemic circulation (e.g., valganciclovir, fosamprenavir, and antibody-, gene-, or virus-directed enzyme prodrugs) [7, 8]. Both types can be further categorized into subtypes depending on whether or not the intracellular converting location is also the site of therapeutic action, or the conversion occurs in the gastrointestinal fluids or systemic circulation. From a regulatory perspective, this new classification system will certainly help in the understanding of a prodrug's pharmacokinetics and safety.
1.2 Basics of Prodrug Design
The design of an appropriate prodrug structure should be considered in the early stages of preclinical development, bearing in mind that prodrugs may alter the tissue distribution, efficacy, and even the toxicity of the parent drug. Although designing a prodrug so as to include all important factors in one molecule is admittedly very challenging, it can still be more feasible than searching for an entirely new therapeutic agent that has the desired properties. Moreover, the prodrug approach can enable the selection of a suitable drug candidate faster. The main factors that should be carefully considered when designing a prodrug structure are as follows:
- Which functional groups on the parent drug are amenable to chemical derivatization?
- Chemical modifications made to the parent drug must be reversible and allow the prodrug to be converted back into the parent drug by an in vivo chemical and/or enzymatic reaction.
- The promoiety should be safe and rapidly excreted from the body. The choice of promoiety and relative safety should be considered with respect to the disease state, the dose, and the duration of therapy.
- The absorption, distribution, metabolism, and excretion (ADME) properties of parent drug and prodrug require a comprehensive understanding.
- Possible degradation by-products can affect both chemical and physical stability that lead to the formation of new degradation products.
Arguably, the most common approaches for prodrug design are aimed at prodrugs undergoing metabolic bioconversion to the active parent molecule by functionally prominent and diversity-tolerant hydrolase enzymes such as peptidases, phosphatases, and, especially, carboxylesterases [9]. Because they are distributed throughout the body, the potential for carboxylesterases to become saturated or the potential for their substrates to become involved in drug–drug interactions is generally insignificant [10], but not unprecedented [11]. Although esterases in general provide a good starting point for prodrug design strategy, premature bioconversion by first-pass metabolism may hinder the success of prodrugs that rely on esterase activation. Moreover, in vitro assessments of the hydrolysis rates are not always good predictors of the relative rates of the in vivo conversion of a prodrug because of confounding physiological processes that cannot be completely controlled in such studies. Cytochrome P450 (CYP450) enzymes, which are prominent in the liver and are also present in the intestine and lung, have been both intentional [12–14] and unintentional [15] targets for some prodrug strategies; however, these enzymes are not as reliable as esterases in prodrug design due to individual variations in liver functions. Finally, there is a growing interest to design prodrugs that are devoid of a detachable promoiety; in other words, bioprecursor prodrugs that are activated by oxidative or reductive metabolism [16, 17]. Figure 1.2 illustrates prodrug structures for the most common parent drug functionalities. Further discussion of functional group approaches in prodrug design, with representative examples, is given in Chapter 3.
Figure 1.2 Common functional groups amenable to prodrug design. Most prodrugs require “synthetic handles,” which are typically heteroatomic combinations.
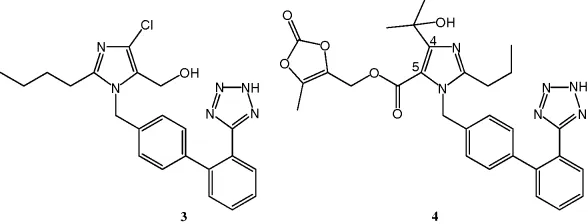
1.3 Rationale for Prodrug Design
Drug discovery is an exceedingly complex and demanding enterprise. During the drug discovery process, new molecular entities (NMEs) are identified by using various techniques that include rational and receptor-based drug design, combinatorial chemistry, and high-throughput screens, or isolating and characterizing active components from living organisms, such as plants, fungi, or bacteria. These technologies can produce novel lead structures with high pharmacological potency. However, until the mid-1990s these technologies frequently ignored important physicochemical and biopharmaceutical aspects of the discovered molecules. These classical drug discovery paradigms often led to drug candidates with poor “drug-like” properties and faced significant problems in later drug development [18, 19]. The term “drug-like” is defined as those compounds that have sufficiently acceptable absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties [19, 20]. Poor outcome resulted in a high attrition rate of potential drug candidates in initial clinical studies when ADMET parameters were not thoroughly studied during preclinical phases. This poor outcome eventually prompted an emphasis to prioritize ADMET-related parameters into the HTS format at earlier decision points, which has enabled the optimization of lead compounds for ADMET properties during the early stages of drug discovery.
The process of developing a prodrug is now more focused also on optimizing the ADMET properties of potential pharmacological compounds, which consequently increases the eventual utility of potential drug candidates [1, 21–26]. Some of the main barriers, which are not limited to a drug's ADMET properties yet may be overcome by a prodrug modification, are listed in Table 1.1 [4, 24]. It should be understood that these obstacles are often intertwined. Several of these issues are also briefly discussed in the following sections.
Table 1.1 Prodrugs can be used to address the following barriers to a drug's usefulness
| Formulation and administration |
| • Inadequate aqueous solubility for liquid dosage forms |
| • Inadequate shelf life for solid or liquid dosage forms |
| • Pain or irritation after local administration∗ |
| • Unpleasant taste or odor |
| Absorption |
| • Inadequate dissolution rate due to low aqueous solubility |
| • Poor membrane permeation and low oral or topical (e.g., dermal and ocular) bioavailability due to poor lipophilicity |
| • Inadequate stability in acidic gastric juices or during first-pass metabolism |
| • Inadequate availability due to efflux mechanisms |
| Distribution |
| • Lack of site specificity (e.g., poor brain or tumor targeting)∗ |
| • Need to decrease plasma protein binding or deposition in lipophilic compartments |
| Metabolism and excretion |
| • Lack or need of site-specific bioactivation∗ |
| • Short duration of action |
| Toxicity |
| • See entries above marked with asterisk∗ (typically associated with lack of site specificity) |
| • Need to temporarily mask a reactive, inherently active, functional group |
| Life cycle management |
| • Development of a prodrug from an existing drug, to achieve improved properties that may represent a life cycle management opportunity. |
1.3.1 Overcoming Formulation and Administration Problems
Sufficient aqueous solubility of a drug is a prerequisite for the preparation of aqueous-based solutions for parenteral or injectable drug dosing. When conventional formulation techniques are not always successful or even possible, such as salt formation, a prodrug strategy becomes an extremely valuable option. From a technical and a commercial point of view, there are several successful prodrugs with improved aqueous solubility properties that serve as solved examples of formulation and administration problems with parenteral administration [27, 28]. The most common approach has been to increase water solubility by introducing an ionizable/polar promoiety to the parent drug. A number of phosphoric acid esters...
Table of contents
- Cover
- Methods and Principles in Medicinal Chemistry
- Title Page
- Copyright
- List of Contributors
- Preface
- A Personal Foreword
- Part One: Prodrug Design and Intellectual Property
- Part Two: Prodrugs Addressing ADMET Issues
- Part Three: Codrugs and Soft Drugs
- Part Four: Preclinical and Clinical Consideration for Prodrugs
- Index