
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
The latest edition of this highly acclaimed textbook, provides a comprehensive and up-to-date overview of the science and medical applications of biopharmaceutical products. Biopharmaceuticals refers to pharmaceutical substances derived from biological sources, and increasingly, it is synonymous with 'newer' pharmaceutical substances derived from genetic engineering or hybridoma technology.
This superbly written review of the important areas of investigation in the field, covers drug production, plus the biochemical and molecular mechanisms of action together with the biotechnology of major biopharmaceutical types on the market or currently under development. There is also additional material reflecting both the technical advances in the area and detailed information on key topics such as the influence of genomics on drug discovery.
This superbly written review of the important areas of investigation in the field, covers drug production, plus the biochemical and molecular mechanisms of action together with the biotechnology of major biopharmaceutical types on the market or currently under development. There is also additional material reflecting both the technical advances in the area and detailed information on key topics such as the influence of genomics on drug discovery.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
Pharmaceuticals, biologics and biopharmaceuticals
INTRODUCTION TO PHARMACEUTICAL PRODUCTS
Pharmaceutical substances form the backbone of modern medicinal therapy. Most traditional pharmaceuticals are low molecular mass organic chemicals (Table 1.1). Although some (e.g. aspirin) were originally isolated from biological sources, most are now manufactured by direct chemical synthesis. Two types of manufacturing companies thus comprise the ‘traditional’ pharmaceutical sector; the chemical synthesis plants, which manufacture the raw chemical ingredients in bulk quantities, and the finished product pharmaceutical facilities, which purchase these raw bulk ingredients, formulate them into final pharmaceutical products, and supply these products to the end-user.
In addition to chemical-based drugs, a range of pharmaceutical substances (e.g. hormones and blood products) are produced by or extracted from biological sources. Such products, some major examples of which are listed in Table 1.2, may thus be described as products of biotechnology. In some instances, categorizing pharmaceuticals as products of biotechnology or chemical synthesis becomes somewhat artificial, e.g. certain semi-synthetic antibiotics are produced by chemical modification of natural antibiotics produced by fermentation technology.
BIOPHARMACEUTICALS AND PHARMACEUTICAL BIOTECHNOLOGY
Terms such as ‘biologic’, ‘biopharmaceutical’ and ‘products of pharmaceutical biotechnology’ or ‘biotechnology medicines’ have now become an accepted part of the pharmaceutical literature. However, these terms are sometimes used interchangeably and can mean different things to different people.
While it might be assumed that ‘biologic’ refers to any pharmaceutical product produced by biotechnological endeavour, its definition is more limited. In pharmaceutical circles, ‘biologic’ generally refers to medicinal products derived from blood, as well as vaccines, toxins and allergen products. Thus, some traditional biotechnology-derived pharmaceutical products (e.g. hormones, antibiotics and plant metabolites) fall outside the strict definition.
Table 1.1. Some traditional pharmaceutical substances which are generally produced by direct chemical synthesis
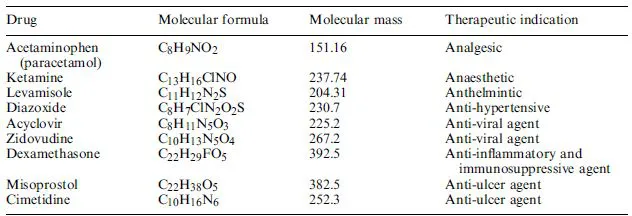
Table 1.2. Some pharmaceuticals which were traditionally obtained by direct extraction from biological source material. Many of the protein-based pharmaceuticals mentioned below are now also produced by genetic engineering
| Blood products (e.g. coagulation factors) | Treatment of blood disorders such as haemophilia A or B |
| Vaccines | Vaccination against various diseases |
| Antibodies | Passive immunization against various diseases |
| Insulin | Treatment of diabetes mellitus |
| Enzymes | Thrombolytic agents, digestive aids, debriding agents (i.e. cleansing of wounds) |
| Antibiotics | Treatment against various infectious agents |
| Plant extracts (e.g. alkaloids) | Various, including pain relief |
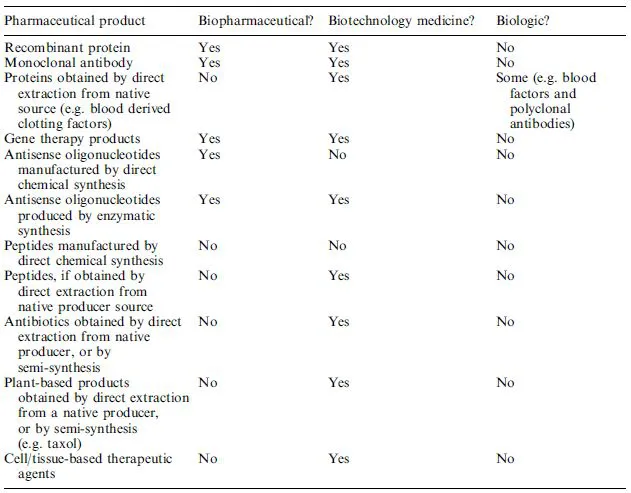
The term ‘biopharmaceutical’ was first used in the 1980s and came to describe a class of therapeutic protein produced by modern biotechnological techniques, specifically via genetic engineering or (in the case of monoclonal antibodies) by hybridoma technology. This usage equated the term ‘biopharmaceutical’ with ‘therapeutic protein synthesized in engineered (non-naturally occurring) biological systems’. More recently, however, nucleic acids used for purposes of gene therapy and antisense technology (Chapter 11) have come to the fore and they too are generally referred to as ‘biopharmaceuticals’. Moreover, several recently approved proteins are used for in vivo diagnostic as opposed to therapeutic purposes. Throughout this book therefore, the term ‘biopharmaceutical’ refers to protein or nucleic acid based pharmaceutical substances used for therapeutic or in vivo diagnostic purposes, which are produced by means other than direct extraction from natural (non-engineered) biological sources (Tables 1.3 and 1.4).
As used herein, ‘biotechnology medicines’ or ‘products of pharmaceutical biotechnology’ are afforded a much broader definition. Unlike the term ‘biopharmaceutical’, the term ‘biotechnology’ has a much broader and long-established meaning. Essentially, it refers to the use of biological systems (e.g. cells or tissues) or biological molecules (e.g. enzymes or antibodies) for or in the manufacture of commercial products. Therefore, the term is equally applicable to long-established biological processes, such as brewing, and more modern processes, such as genetic engineering. As such, the term ‘biotechnology medicine’ is defined here as ‘any pharmaceutical product used for a therapeutic or in vivo diagnostic purpose, which is produced in full or in part by either traditional or modern biotechnological means’. Such products encompass, for example, antibiotics extracted from fungi, therapeutic proteins extracted from native source material (e.g. insulin from pig pancreas) and products produced by genetic engineering (e.g. recombinant insulin) (Tables 1.3 and 1.4).
Table 1.3. A summary of the definition of the terms ‘biologic’, ‘biopharmaceutical’ and ‘biotechnology medicine’ as used throughout this book. Reprinted from European Journal of Pharmaceutical Sciences, vol 15, Walsh, Biopharmaceuticals and Biotechnology, p 135–138, ©2002, with permission from Elsevier Science
| Biopharmaceutical | A protein or nucleic acid based pharmaceutical substance used for therapeutic or in vivo diagnostic purposes, which is produced by means other than direct extraction from a native (non-engineered) biological source |
| Biotechnology medicine/product of pharmaceutical biotechnology | Any pharmaceutical product used for therapeutic or in vivo diagnostic purposes, which is produced in full or in part by biotechnological means |
| Biologic | A virus, therapeutic serum, toxin, antitoxin, vaccine, blood, blood component or derivative, allergenic product or analogous product, or arsphenamine or its derivatives or any other trivalent organic arsenic compound applicable to the prevention, cure or treatment of disease or conditions of human beings |
HISTORY OF THE PHARMACEUTICAL INDUSTRY
The pharmaceutical industry, as we now know it, is barely 60 years old. From very modest beginnings it has grown rapidly, reaching an estimated value of $100 billion by the mid-1980s. Its current value is likely double this figure or more. There are well in excess of 10 000 pharmaceutical companies in existence, although only about 100 of these can claim to be of true international significance. These companies manufacture in excess of 5000 individual pharmaceutical substances used routinely in medicine.
The first stages of development of the modern pharmaceutical industry can be traced back to the turn of the twentieth century. At that time (apart from folk cures), the medical community had at their disposal only four drugs that were effective in treating specific diseases:
- Digitalis, extracted from foxglove, was known to stimulate heart muscle and hence was used to treat various heart conditions.
- Quinine, obtained from the barks/roots of a plant (Cinchona sp.), was used to treat malaria.
- Pecacuanha (active ingredient is a mixture of alkaloids), used for treating dysentery, was obtained from the bark/roots of the plant species Cephaelis.
- Mercury, for the treatment of syphilis.
Table 1.4. The categorization of pharmaceutically significant biological molecules using the indicated definitions as listed in Table 1.3. Reproduced in modified form from European Journal of Pharmaceutical Sciences, vol 15, Walsh, Biopharmaceuticals and Biotechnology, p 135–138, ©2002, with permission from Elsevier Science
The lack of appropriate safe and effective medicines contributed in no small way to the low life expectancy characteristic of those times.
Developments in biology (particularly the growing realization of the microbiological basis of many diseases), as well as a developing appreciation of the principles of organic chemistry, helped underpin future innovation in the fledgling pharmaceutical industry. The successful synthesis of various artificial dyes, which proved to be therapeutically useful, led to the formation of pharmaceutical/chemical companies such as Bayer and Hoechst in the late 1800s, e.g. scientists at Bayer succeeded in synthesizing aspirin in 1895.
Despite these early advances, it was not until the 1930s that the pharmaceutical industry began to develop in earnest. The initial landmark discovery of this era was probably the discovery and chemical synthesis of the sulpha drugs. These are a group of related molecules derived from the red dye, Prontosil rubrum. These drugs proved effective in the treatment of a wide variety of bacterial infections (Figure 1.1). Although it was first used therapeutically in the early 1920s, large-scale industrial production of insulin also commenced in the 1930s.
The medical success of these drugs gave new emphasis to the pharmaceutical industry, which was boosted further by the commencement of industrial-scale penicillin manufacture in the early 1940s. Around this time, many of the current leading pharmaceutical companies (or their forerunners) were founded. Examples include Ciba Geigy, Eli Lilly, Wellcome, Glaxo and Roche. Over the next two to three decades, these companies developed drugs such as tetracyclines, corticosteroids, oral contraceptives, antidepressants and many more. Most of these pharmaceutical substances are manufactured by direct chemical synthesis.
THE AGE OF BIOPHARMACEUTICALS
Biomedical research continues to broaden our understanding of the molecular mechanisms underlining both health and disease. Research undertaken since the 1950s has pinpointed a host of proteins produced naturally in the body which have obvious therapeutic applications. Examples include the interferons, and interleukins, which regulate the immune response; growth factors such as erythropoietin, which stimulates red blood cell production; and neurotrophic factors, which regulate the development and maintenance of neural tissue.
While the pharmaceutical potential of these regulatory molecules was generally appreciated, their widespread medical application was in most cases rendered impractical due to the tiny quantities in which they were naturally produced. The advent of recombinant DNA technology (genetic engineering) and monoclonal antibody technology (hybridoma technology) overcame many such difficulties, and marked the beginning of a new era of the pharmaceutical sciences.
Recombinant DNA technology has had a four-fold positive impact upon the production of pharmaceutically important proteins:
- It overcomes the problem of source availability. Many proteins of therapeutic potential are produced naturally in the body in minute quantities. Examples include interferons (Chapter 4), interleukins (Chapter 5) and colony stimulating factors (Chapter 6). This rendered impractical their direct extraction from native source material in quantities sufficient to meet likely clinical demand. Recombinant production (Chapter 3) allows the manufacture of any protein in whatever quantity it is required.
- It overcomes problems of product safety. Direct extraction of product from some native biological sources has, in the past, led to the unwitting transmission of disease. Examples include the transmission of blood-borne pathogens such as hepatitis B, C and HIV via infected blood products and the transmission of Creutzfeldt–Jakob disease to persons receiving human growth hormone preparations derived from human pituitaries.
- It provides an alternative to direct extraction from inappropriate/dangerous source material. A number of therapeutic proteins have traditionally been extracted from human urine. The fertility hormone FSH, for example, is obtained from the urine of post-menopausal women, while a related hormone, hCG, is extracted from the urine of pregnant women (Chapter 8). Urine is not considered a particularly desirable source of pharmaceutical products. While several products obtained from this source remain on the market, recombinant forms have now also been approved. Other potential biopharmaceuticals are produced naturally in downright dangerous sources. Ancrod, for example, is a protein displaying anti-coagulant activity (Chapter 9) and, hence, is of potential clinical use; however, it is produced naturally by the Malaysian pit viper. While retrieval by milking snake venom is possible, and indeed may be quite a...
Table of contents
- Cover
- Contents
- Title Page
- Copyright
- Preface
- Chapter 1: Pharmaceuticals, biologics and biopharmaceuticals
- Chapter 2: The drug development process
- Chapter 3: The drug manufacturing process
- Chapter 4: The cytokines—the interferon family
- Chapter 5: Cytokines: interleukins and tumour necrosis factor
- Chapter 6: Haemopoietic growth factors
- Chapter 7: Growth factors
- Chapter 8: Hormones of therapeutic interest
- Chapter 9: Blood products and therapeutic enzymes
- Chapter 10: Antibodies, vaccines and adjuvants
- Chapter 11: Nucleic acid therapeutics
- Appendix 1: Biopharmaceuticals thus far approved in the USA or European Union
- Appendix 2: Some Internet addresses relevant to the biopharmaceutical sector
- Appendix 3: Two selected monographs reproduced from the European Pharmacopoeia with permission from the European Commission*
- Appendix 4 Annex 2 ‘Manufacture of biological medicinal products for human use.’1
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Biopharmaceuticals by Gary Walsh in PDF and/or ePUB format, as well as other popular books in Medicine & Pharmacology. We have over one million books available in our catalogue for you to explore.