
Thermodynamics for the Practicing Engineer
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Thermodynamics for the Practicing Engineer
About this book
Enables you to easily advance from thermodynamics principles to applications
Thermodynamics for the Practicing Engineer, as the title suggests, is written for all practicing engineers and anyone studying to become one. Its focus therefore is on applications of thermodynamics, addressing both technical and pragmatic problems in the field. Readers are provided a solid base in thermodynamics theory; however, the text is mostly dedicated to demonstrating how theory is applied to solve real-world problems.
This text's four parts enable readers to easily gain a foundation in basic principles and then learn how to apply them in practice:
- Part One: Introduction. Sets forth the basic principles of thermodynamics, reviewing such topics as units and dimensions, conservation laws, gas laws, and the second law of thermodynamics.
-
Part Two: Enthalpy Effects. Examines sensible, latent, chemical reaction, and mixing enthalpy effects.
-
Part Three: Equilibrium Thermodynamics. Addresses both principles and calculations for phase, vapor-liquid, and chemical reaction equilibrium.
-
Part Four: Other Topics. Reviews such important issues as economics, numerical methods, open-ended problems, environmental concerns, health and safety management, ethics, and exergy.
Throughout the text, detailed illustrative examples demonstrate how all the principles, procedures, and equations are put into practice. Additional practice problems enable readers to solve real-world problems similar to the ones that they will encounter on the job.
Readers will gain a solid working knowledge of thermodynamics principles and applications upon successful completion of this text. Moreover, they will be better prepared when approaching/addressing advanced material and more complex problems.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
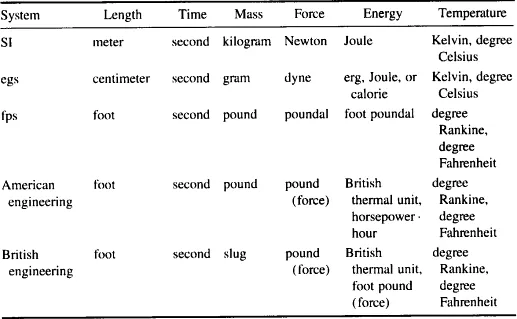
| Physical quantity | Name of unit | Symbol for unit |
| Length | foot | ft |
| Time | second | s |
| Mass | pound (mass) | lb |
| Temperature | degree Rankine | °R |
| Temperature (alternative) | degree Fahrenheit | °F |
| Moles | pound · mole | lbmol |
| Energy | British thermal unit | Btu |
| Energy (alternative) | horsepower · hour | hp · h |
| Force | pound (force) | lbf |
| Acceleration | foot per second square | ft/s2 |
| Velocity | foot per second | ft/s |
| Volume | cubic foot | ft3 |
| Area | square foot | ft2 |
| Frequency | cycles per second, hertz | cycles/s, Hz |
| Power | horsepower, Btu per second | hp, Btu/s |
| Heat capacity | British thermal unit per (pound mass · degree Rankine) | Btu/lb · °R |
| Density | pound (mass) per cubic foot | lb/ft3 |
| Pressure | pound (force) per square inch | psi |
| pound (force... |
Table of contents
- Cover
- Half Title page
- Title page
- Copyright page
- Dedication
- Preface
- Part I: Introduction
- Part II: Enthalpy Effects
- Part III: Equilibrium Thermodynamics
- Part IV: Other Topics
- Appendix
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app