
Membranes for Membrane Reactors
Preparation, Optimization and Selection
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Membranes for Membrane Reactors
Preparation, Optimization and Selection
About this book
A membrane reactor is a device for simultaneously performing a reaction and a membrane-based separation in the same physical device. Therefore, the membrane not only plays the role of a separator, but also takes place in the reaction itself.
This text covers, in detail, the preparation and characterisation of all types of membranes used in membranes reactors. Each membrane synthesis process used by membranologists is explained by well known scientists in their specific research field.
The book opens with an exhaustive review and introduction to membrane reactors, introducing the recent advances in this field. The following chapters concern the preparation of both organic and inorganic, and in both cases, a deep analysis of all the techniques used to prepare membrane are presented and discussed. A brief historical introduction for each technique is also included, followed by a complete description of the technique as well as the main results presented in the international specialized literature. In order to give to the reader a summary look to the overall work, a conclusive chapter is included for collecting all the information presented in the previous chapters.
Key features:
- Fills a gap in the market for a scientific book describing the preparation and characterization of all the kind of membranes used in membrane reactors
- Discusses an important topic - there is increasing emphasis on membranes in general, due to their use as energy efficient separation tools and the 'green' chemistry opportunities they offer
- Includes a review about membrane reactors, several chapters concerning the preparation organic, inorganic, dense, porous, and composite membranes and a conclusion with a comparison among the different membrane preparation techniques
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
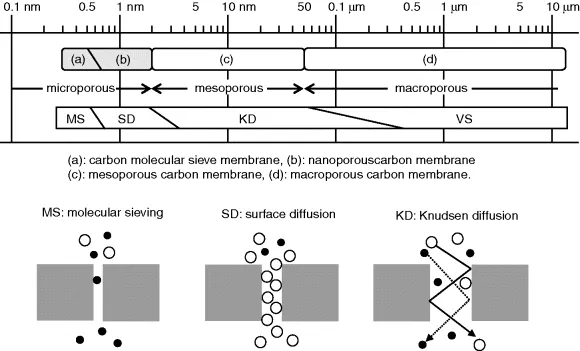
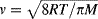
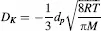


Table of contents
- Cover
- Title Page
- Copyright
- Contributors
- Glossary
- Introduction – A Review of Membrane Reactors
- Chapter 1: Microporous Carbon Membranes
- Chapter 2: Metallic Membranes by Wire Arc Spraying: Preparation, Characterisation and Applications
- Chapter 3: Inorganic Hollow Fibre Membranes for Chemical Reaction
- Chapter 4: Metallic Membranes Prepared by Cold Rolling and Diffusion Welding
- Chapter 5: Preparation and Synthesis of Mixed Ionic and Electronic Conducting Ceramic Membranes for Oxygen Permeation
- Chapter 6: Nanostructured Perovskites for the Fabrication of Thin Ceramic Membranes and Related Phenomena
- Chapter 7: Nanostructured Perovskites for the Fabrication of Thin Ceramic Membranes and Related Phenomena
- Chapter 8: Zeolite Membrane Reactors
- Chapter 9: Metal Supported and Laminated Pd-Based Membranes
- Chapter 10: PVD Techniques for Metallic Membrane Reactors
- Chapter 11: Membranes Prepared via Electroless Plating
- Chapter 12: Silica Membranes – Preparation by Chemical Vapour Deposition and Characteristics
- Chapter 13: Membranes Prepared via Molecular Layering Method
- Chapter 14: Solvated Metal Atoms in the Preparation of Catalytic Membranes
- Chapter 15: Electrophoretic Deposition for the Synthesis of Inorganic Membranes
- Chapter 16: Electrochemical Preparation of Nanoparticle Deposits: Application to Membranes and Catalysis
- Chapter 17: Electrochemical Preparation of Pd Seeds/Inorganic Multilayers on Structured Metallic Fibres
- Chapter 18: Membranes Prepared Via Spray Pyrolysis
- Chapter 19: Silica Membranes – Preparation and Characterisation of Nanocrystalline and Quasicrystalline Alloys by Planar Flow Casting for Metal Membranes
- Chapter 20: Silica Membranes – Preparation and Characterisation of Amorphous Alloy Membranes
- Chapter 21: Membranes Prepared Via Phase Inversion
- Chapter 22: Porous Flat Sheet, Hollow Fibre and Capsule Membranes by Phase Separation of Polymer Solutions
- Chapter 23: Porous Polymer Membranes by Manufacturing Technologies other than Phase Separation of Polymer Solutions
- Chapter 24: Palladium-Loaded Polymeric Membranes for Hydrogenation in Catalytic Membrane Reactors
- Chapter 25: Membrane Prepared via Plasma Modification
- Chapter 26: Enzyme-Immobilised Polymer Membranes for Chemical Reactions
- Final Remarks
- Color Plates
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app