![]()
Chapter 1
An overview of the chemistry, manufacture, environmental fate and detection of carbofuran
Stephen Donovan,1 Mark Taggart,2 Ngaio Richards3,4
1Pennsylvania Department of Health, 110 Pickering Way, Lionville, Pennsylvania 19353, USA
2Environmental Research Institute, University of the Highlands and Islands, Castle Street, Thurso, Caithness, Scotland, KW14 7JD, UK
3Working Dogs for Conservation, 52 Eustis Road, Three Forks, Montana 59752, USA
4Department of Life Sciences, Anglia Ruskin University, East Road, Cambridge CB1 1PT, UK
1.1 Introduction
The aim of this chapter is to provide the reader with a comprehensive understanding of carbofuran as a compound and a familiarity with the technical terms used throughout this book. First, we outline the features which differentiate carbofuran from other compounds and detail its chemical properties. We then summarise its environmental fate, in other words what happens to it once it is in the environment, and conclude with a discussion of the most common methods of analysing and detecting carbofuran in environmental samples.
1.2 The chemistry and mode of action of carbofuran
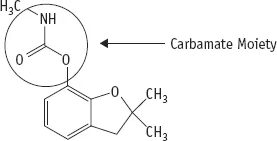
Carbofuran is an organic compound (meaning that it is made up of a carbon skeleton), composed of a benzofuranyl component which is connected to a carbamate group (circled in Figure 1.1), i.e., derived from carbamic acid. Its molecular formula is denoted as C12H15NO3 and its chemical name is: 2,3-dihydro-2,2-dimethyl-7-benzofuranyl N-methylcarbamate. Carbofuran is a systemic insecticide, which means that when it is applied it enters into a plant, is transported by the sap, and when insects or other pests feed on other parts of the plant, they become poisoned.
The chemical structure of carbofuran is shown in Figure 1.1. As a group, carbamates can be classified into N-methyl carbamates of phenols (e.g., carbofuran, carbaryl (Figure 1.5) and propoxur) and the N-methyl carbamates of oximes (e.g., aldicarb and methomyl). These carbamates can be synthesised from the reaction of methyl isocyanate with the hydroxyl group of phenols and oximes. The biological activity of these carbamates comes from their ability to essentially liberate methyl isocyanate (MIC) inside the organism. Methyl isocyanate is quite reactive (i.e., toxic) and binds to enzymes that have reactive sulfhydro (RSH) and hydroxy (OH) groups. Since the activity of enzymes often relies on such groups repeatedly making and breaking bonds many thousand of times a second, the enzymes become inactive (inhibited). MIC is the industrial compound that was released into the air in 1984 in Bhopal (India) and caused the death of between 3 000 and 15 000 people and injured over half a million people (see also Chapter 4).
Other important pesticide groups include the organophosphorus pesticides (e.g., monocrotophos (Figure 1.6), dimethoate, diazinon and phosalone), and the organochlorines (e.g., DDT (Figure 1.7), aldrin, its metabolite dieldrin (Figure 1.8), and endrin), often abbreviated as ‘OP/OPCs’ or ‘OCs’, respectively. Carbamates (often abbreviated as ‘CMs’ or ‘CBs’) and organophosphorus compounds both have a non-discriminate (or broad-spectrum) mode of action, i.e., one that inhibits cholinesterase enzyme activity in insects, mammals and birds. For this reason they are sometimes referred to as ‘anti-cholinesterases’. Involved in virtually all physiological responses and mechanisms, no other enzyme is thought to perform such a complex or extensive set of functions within the animal kingdom. The mechanism by which cholinesterase inhibition occurs and its clinical impact on avian and mammalian wildlife, are further detailed in Chapter 2, which also discusses relevant diagnostic and rehabilitation measures.
It is this broad spectrum of activity that also makes carbofuran an ideal insecticide, acaricide (against ticks and mites) and nematicide (against nematodes). Plant protection products containing carbofuran as the active ingredient (often denoted as ‘ai’ or ‘AI’) have been used worldwide to control pests in sugarcane, sugar beet, maize, coffee and rice crops. Carbofuran is available in liquid, silica-based granular and corncob formulations (further discussed in Chapter 8). Sand, clay or granulated dried corncob formulations are intended to enable the active ingredient to be released more slowly into the rhizosphere, the zone immediately surrounding the roots of a developing plant.
As such, carbofuran is particularly effective in controlling rice pests such as green leafhoppers (Nephotettix virescens), brown plant hoppers (Nilaparvata lugens) and more generally, stem borers and whorl maggots. This is because leaf hoppers and plant hoppers are piercing-sucking phloem feeders, and carbofuran is phloem systemic and therefore available in the phloem sap. Other pests, even if resistant to organophosphorus insecticides (e.g., white flies, leaf miners, ants, scale insects, cockroaches, wasps and aphids), can be effectively controlled by carbofuran. Although both organophosphates and carbamates have the same mode of action, different organisms can be resistant to one class of compound but not necessarily resistant to the other.
Unfortunately, for reasons which remain unclear, birds in particular are simply not equipped to detoxify (or effectively metabolise) either carbamate or organophosphorus compounds before succumbing to their toxic effect (Mineau 2009). Consequently, such ‘general biocides’ are now increasingly viewed as ‘old-fashioned’ and, as such, are very slowly being phased out and replaced by new compounds that do discriminate between target insects and non-target organisms or wildlife. Such compounds are therefore inherently less ‘ecotoxic’, but may still have significant unintended impacts on beneficial non-target insects, among others. For example, imidacloprid, a nicotinic systemic insecticide, was introduced as a ‘less toxic’ replacement. While not very toxic to animals in general (see www.pesticidemanual.com/ and http://www.beekeeping.com/articles/us/imidacloprid_bayer.htm), studies have indicated that exposure to sublethal levels slows mobility and communication capacity in honeybees (e.g., Medrzycki, Montanari, Bortolotti et al. 2003).
1.3 Manufacture and formulation of carbofuran
Carbofuran was developed in the 1960s, patented in 1965 (Budavari 1989), and introduced on the market as a systemic and broad spectrum nematicide in 1967 under the well-known brand/trade name of Furadan by FMC (Farm Machinery Corporation), based in Philadelphia in the United States (http://www.fmc.com/AboutFMC/CorporateOverview/FMCHistory.aspx?PageContentID=9). In some parts of this book (especially Chapter 3, regarding the situation in Kenya), the ‘names’ carbofuran and Furadan are effectively used interchangeably. This simply reflects the fact that in certain countries carbofuran (i.e., the product name) is more commonly known by its trade/brand name (in this case, Furadan). Each formulation is named according to its percentage active ingredient, i.e., the amount of carbofuran (by weight) in the formulation. Hence, Furadan 3G, 10G, and 15G contain 3, 10 and 15% (w/w, i.e., weight by weight or wet weight) of the active ingredient, respectively.
FMC held sole patent from the 1960s and is still considered the major global manufacturer of carbofuran. Patent law is country-specific, and we were unable to specifically determine when FMC’s original patent would have expired (i.e., when generic formulations would have been permitted). However, in the United States, patents are granted for a maximum of 20 years, and Chapters 5 and 6, for example, list other manufacturers as registrants of the product in the late 1980s, which leads us to believe that FMC’s sole patent expired sometime in the mid to late 1980s. Table 1.1 lists other known manufacturers of carbofuran around the world. For a complete list of manufacturers of carbofuran products, the reader is referred to the Pesticide Manual (www.pesticidemanual.com/).
Table 1.1 Name and headquarter location of selected companies that manufacture carbofuran products and trade names under which they are sold
Information taken from The Pesticide Manual (www.pesticidemanual.com/)
| Company name | Headquarter location | Trade name |
| Agro-Chemie | Hungary | Chinufur |
| Aimco Pesticides Limited | India | Furacarb |
| Cequisa | Spain | Cekufuran |
| Makhteshim Agan Industries | Israel | Carbodan |
| Nagajura Agridar | India | Fury |
| Sanachem (Pty) Ltd | South Africa | Terrafuran |
| Sipcam | Italy | Carbosip, Rampart |
| Sanonda/Zhengzhou Pesticides Co Ltd. | China | Agrofuran |
1.4 Carbofuran in the environment
The dominant source of carbofuran emission to the environment is via its application as an insecticide. In this context, it is sobering to consider that, in general, approximately 90% of all agricultural pesticide applications never actually reach their target organism(s). This ‘excess’ is instead widely dispersed into the environment, entering the air, soil and water (Moses, Johnson and Auger 1993). The environmental fate and persistence of any specific compound is also governed by the prevailing climate and as such differs between tropical and temperate regions (Fodor-Csorba 1998). Elevated temperatures can lead to pesticide loss and deterioration through volatilisation (i.e., transformation to a gas and then dissipation) and increased microbial activity. Sunlight and ultraviolet (UV) intensity is also greater in tropical and subtropical regions, which again can lead to more rapid photodegradation (Fodor-Csorba 1998). Such degradation and the reaction products formed (some of which may be more toxic than the original parent compound) are then themselves transported into the environment. The ability to identify and analyse such degradation products and metabolites is likely to become increasingly important in the future as ‘sustainable’ biocide products with low ecotoxicity are identified and developed.
In soil, chemical transformation processes are influenced by factors such as pH, temperature, clay content, organic matter content, moisture content, the presence of micro-organisms, and the types of functional groups that are attached to the pesticide molecule (Lalah, Kaigwara, Getenga et al. 2001). Chemical reactions can be catalysed by clay surfaces, metal oxides and metal ions in soil. Likewise, the rate of chemical hydrolysis (i.e., the addition of water to a compound) ...