
- English
- ePUB (mobile friendly)
- Available on iOS & Android
An Introduction to Chemical Kinetics
About this book
This book is a progressive presentation of kinetics of the chemical reactions. It provides complete coverage of the domain of chemical kinetics, which is necessary for the various future users in the fields of Chemistry, Physical Chemistry, Materials Science, Chemical Engineering, Macromolecular Chemistry and Combustion. It will help them to understand the most sophisticated knowledge of their future job area.
Over 15 chapters, this book present the fundamentals of chemical kinetics, its relations with reaction mechanisms and kinetic properties. Two chapters are then devoted to experimental results and how to calculate the kinetic laws in both homogeneous and heterogeneous systems. The following two chapters describe the main approximation modes to calculate these laws. Three chapters are devoted to elementary steps with the various classes, the principles used to write them and their modeling using the theory of the activated complex in gas and condensed phases. Three chapters are devoted to the particular areas of chemical reactions, chain reactions, catalysis and the stoichiometric heterogeneous reactions. Finally the non-steady-state processes of combustion and explosion are treated in the final chapter.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Basic Concepts of Chemical Kinetics
Chapter 1
Chemical Reaction and Kinetic Quantities
1.1. The chemical reaction
1.1.1. The chemical equation and stoichiometric coefficients
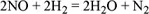
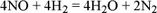
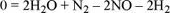
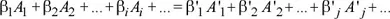
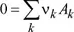
Table of contents
- Cover
- Title Page
- Copyright
- Preface
- Part 1: Basic Concepts of Chemical Kinetics
- Part 2: Reaction Mechanisms and Kinetic Properties
- Appendix
- Notations and Symbols
- Bibliography
- Index